Tissue proteomics of the human mammary gland: towards an abridged definition of the molecular phenotypes underlying epithelial normalcy
- PMID: 21036680
- PMCID: PMC5527921
- DOI: 10.1016/j.molonc.2010.09.005
Tissue proteomics of the human mammary gland: towards an abridged definition of the molecular phenotypes underlying epithelial normalcy
Abstract
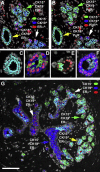
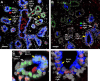
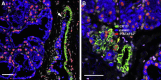
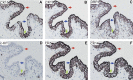
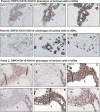
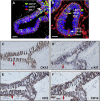
Our limited understanding of the biological impact of the whole spectrum of early breast lesions together with a lack of accurate molecular-based risk criteria for the diagnosis and assignment of prognostic significance to biopsy findings presents an important problem in the clinical management of patients harboring precancerous breast lesions. As a result, there is a need to identify biomarkers that can better determine the outcome of early breast lesions by identifying subpopulations of cells in breast premalignant disease that are at high-risk of progression to invasive disease. A first step towards achieving this goal will be to define the molecular phenotypes of the various cell types and precursors - generated by the stem cell hierarchy - that are present in normal and benign conditions of the breast. To date there have been very few systematic proteomic studies aimed at characterizing the phenotypes of the different cell subpopulations present in normal human mammary tissue, partly due to the formidable heterogeneity of mammary tissue, but also due to limitations of the current proteomic technologies. Work in our laboratories has attempted to address in a systematic fashion some of these limitations and here we present our efforts to search for biomarkers using normal fresh tissue from non-neoplastic breast samples. From the data generated by the 2D gel-based proteomic profiling we were able to compile a protein database of normal human breast epithelial tissue that was used to support the biomarker discovery program. We review and present new data on the putative cell-progenitor marker cytokeratin 15 (CK15), and describe a novel marker, dihydropyriminidase-related protein 3 (DRP3) that in combination with CK15 and other well known proteins were used to define molecular phenotypes of normal human breast epithelial cells and their progenitors in resting acini, lactating alveoli, and large collecting ducts of the nipple. Preliminary results are also presented concerning DRP3 positive usual ductal hyperplasias (UDHs) and on single cell layer columnar cells (CCCs). At least two bona fide biomarkers of undifferentiated ERα/PgR negative luminal cells emerged from these studies, CK15 and c-KIT, which in combination with transformation markers may lead to the establishment of a protein signature able to identify breast precancerous at risk of progressing to invasive disease.
Copyright © 2010 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures






References
-
- Abdel-Fatah, T.M.A. , Powe, D.G. , Hodi, Z. , Lee, A.H.S. , Reis-Filho, J.S. , Ellis, I.O. , 2007. High frequency of coexistence of columnar cell lesions, lobular neoplasia, and low grade ductal carcinoma in situ with invasive tumular carcinoma and invasive lobular carcinoma. Am. J. Surg. Pathol. 31, 417–426. - PubMed
-
- In Altekruse S.F., Kosary C.L., Krapcho M., Neyman N., Aminou R., Waldron W., Ruhl J., Howlader N., Tatalovich Z., Cho H., Mariotto A., Eisner M.P., Lewis D.R., Cronin K., Chen H.S., Feuer E.J., Stinchcomb D.G., Edwards B.K.(Eds.), SEER Cancer Statistics Review, 1975–2007. National Cancer Institute; Bethesda, MD:
-
- Bánkfalvi, A. , Ludwig, A. , De-Hesselle, B. , Buerger, H. , Buchwalow, I.B. , Boecker, W. , 2004. Different proliferative activity of the glandular and myoepithelial lineages in benign proliferative and early malignant breast diseases. Mod. Pathol. 17, 1051–1061. - PubMed
-
- Barbareschi, M. , Pecciarini, L. , Cangi, M.G. , Macrì, E. , Rizzo, A. , Viale, G. , Doglioni, C. , 2001. p63, a p53 homologue, is a selective nuclear marker of myoepithelial cells of the human breast. Am. J. Surg. Pathol. 25, 1054–1060. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

