Continuous administration of sorafenib in combination with transarterial chemoembolization in patients with hepatocellular carcinoma: results of a phase I study
- PMID: 21036880
- PMCID: PMC3227910
- DOI: 10.1634/theoncologist.2010-0180
Continuous administration of sorafenib in combination with transarterial chemoembolization in patients with hepatocellular carcinoma: results of a phase I study
Abstract
Background and aim: It is unknown whether sorafenib can be combined with transarterial chemoembolization (TACE) in patients with hepatocellular carcinoma. This study assesses the safety and tolerability of a continuous regimen of sorafenib combined with TACE.
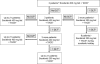
Methods: This was an open-label phase I study testing a continuous administration of sorafenib (dose escalation from 200 mg twice daily [bid] to 400 mg bid) starting 7 days prior to TACE with doxorubicin (50 mg).
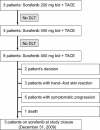
Results: Twenty-one patients were screened and 14 received sorafenib combined with TACE. Because there were no dose-limiting toxicities in the first three patients who received sorafenib at a dose of 200 mg bid, subsequent patients received 400 mg bid. Twenty-seven procedures were performed (median, two per patient) and two local therapy-related severe adverse events occurred. The median duration of sorafenib therapy was 246 days (range, 14-547 days). Sorafenib-related adverse events of grade ≥3 were hand-foot skin reaction (n = 3), weight loss (n = 2), diarrhea (n = 1), abdominal pain (n = 1), and thrombocytopenia (n = 3). After treatment with sorafenib and TACE, there was a significant decrease in the concentration of plasma vascular endothelial growth factor (VEGF) from 93 ng/l to 67 ng/l.
Conclusions: Continuous administration of sorafenib at a dose of 400 mg bid combined with TACE was tolerable. The adverse event profile of this regimen was comparable with that of sorafenib monotherapy with the exception of thrombocytopenia, which may be more frequent. There were no increases in the circulating VEGF levels after TACE with this combined regimen. (Swiss Association for the Study of the Liver study number 25; ClinicalTrials.gov trial identifier, NCT00478374).
Conflict of interest statement
The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias. No financial relationships relevant to the content of this article have been disclosed by the independent peer reviewers.
Figures
References
-
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. - PubMed
-
- Llovet JM, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. - PubMed
-
- Camma C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: Meta-analysis of randomized controlled trials. Radiology. 2002;224:47–54. - PubMed
-
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–442. - PubMed
-
- Li X, Feng GS, Zheng CS, et al. Influence of transarterial chemoembolization on angiogenesis and expression of vascular endothelial growth factor and basic fibroblast growth factor in rat with Walker-256 transplanted hepatoma: An experimental study. World J Gastroenterol. 2003;9:2445–2449. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

