Origin of absorption changes associated with photoprotective energy dissipation in the absence of zeaxanthin
- PMID: 21036900
- PMCID: PMC3013049
- DOI: 10.1074/jbc.M110.184887
Origin of absorption changes associated with photoprotective energy dissipation in the absence of zeaxanthin
Abstract
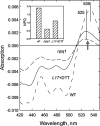
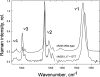
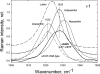
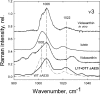
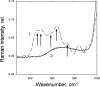
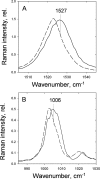
To prevent photo-oxidative damage to the photosynthetic membrane in strong light, plants dissipate excess absorbed light energy as heat in a mechanism known as non-photochemical quenching (NPQ). NPQ is triggered by the trans-membrane proton gradient (ΔpH), which causes the protonation of the photosystem II light-harvesting antenna (LHCII) and the PsbS protein, as well as the de-epoxidation of the xanthophyll violaxanthin to zeaxanthin. The combination of these factors brings about formation of dissipative pigment interactions that quench the excess energy. The formation of NPQ is associated with certain absorption changes that have been suggested to reflect a conformational change in LHCII brought about by its protonation. The light-minus-dark recovery absorption difference spectrum is characterized by a series of positive and negative bands, the best known of which is ΔA(535). Light-minus-dark recovery resonance Raman difference spectra performed at the wavelength of the absorption change of interest allows identification of the pigment responsible from its unique vibrational signature. Using this technique, the origin of ΔA(535) was previously shown to be a subpopulation of red-shifted zeaxanthin molecules. In the absence of zeaxanthin (and antheraxanthin), a proportion of NPQ remains, and the ΔA(535) change is blue-shifted to 525 nm (ΔA(525)). Using resonance Raman spectroscopy, it is shown that the ΔA(525) absorption change in Arabidopsis leaves lacking zeaxanthin belongs to a red-shifted subpopulation of violaxanthin molecules formed during NPQ. The presence of the same ΔA(535) and ΔA(525) Raman signatures in vitro in aggregated LHCII, containing zeaxanthin and violaxanthin, respectively, leads to a new proposal for the origin of the xanthophyll red shifts associated with NPQ.
Figures
Similar articles
-
Acclimation- and mutation-induced enhancement of PsbS levels affects the kinetics of non-photochemical quenching in Arabidopsis thaliana.Planta. 2011 Jun;233(6):1253-64. doi: 10.1007/s00425-011-1380-5. Epub 2011 Feb 22. Planta. 2011. PMID: 21340700
-
The xanthophyll cycle affects reversible interactions between PsbS and light-harvesting complex II to control non-photochemical quenching.Nat Plants. 2017 Jan 30;3:16225. doi: 10.1038/nplants.2016.225. Nat Plants. 2017. PMID: 28134919
-
Restoration of rapidly reversible photoprotective energy dissipation in the absence of PsbS protein by enhanced DeltapH.J Biol Chem. 2011 Jun 3;286(22):19973-81. doi: 10.1074/jbc.M111.237255. Epub 2011 Apr 7. J Biol Chem. 2011. PMID: 21474447 Free PMC article.
-
Mechanism and regulation of the violaxanthin cycle: the role of antenna proteins and membrane lipids.Biochim Biophys Acta. 2009 Jan;1787(1):3-14. doi: 10.1016/j.bbabio.2008.09.013. Epub 2008 Oct 11. Biochim Biophys Acta. 2009. PMID: 18976630 Review.
-
Xanthophylls as modulators of membrane protein function.Arch Biochem Biophys. 2010 Dec 1;504(1):78-85. doi: 10.1016/j.abb.2010.06.034. Epub 2010 Jul 6. Arch Biochem Biophys. 2010. PMID: 20615387 Review.
Cited by
-
Impacts of Drought on Photosynthesis in Major Food Crops and the Related Mechanisms of Plant Responses to Drought.Plants (Basel). 2024 Jun 30;13(13):1808. doi: 10.3390/plants13131808. Plants (Basel). 2024. PMID: 38999648 Free PMC article. Review.
-
Effect of protein aggregation on the spectroscopic properties and excited state kinetics of the LHCII pigment–protein complex from green plants.Photosynth Res. 2013 Dec;118(3):259-76. doi: 10.1007/s11120-013-9924-0. Photosynth Res. 2013. PMID: 24077891
-
A comparison between plant photosystem I and photosystem II architecture and functioning.Curr Protein Pept Sci. 2014;15(4):296-331. doi: 10.2174/1389203715666140327102218. Curr Protein Pept Sci. 2014. PMID: 24678674 Free PMC article. Review.
-
Rethinking the existence of a steady-state Δψ component of the proton motive force across plant thylakoid membranes.Photosynth Res. 2014 Feb;119(1-2):233-42. doi: 10.1007/s11120-013-9817-2. Epub 2013 Mar 29. Photosynth Res. 2014. PMID: 23539362
-
State-transitions facilitate robust quantum yields and cause an over-estimation of electron transport in Dunaliella tertiolecta cells held at the CO₂ compensation point and re-supplied with DIC.Photosynth Res. 2014 Mar;119(3):257-72. doi: 10.1007/s11120-013-9937-8. Epub 2013 Oct 18. Photosynth Res. 2014. PMID: 24135997
References
-
- Dekker J. P., Boekema E. J. (2005) Biochim. Biophys. Acta 1706, 12–39 - PubMed
-
- Ledford H. K., Niyogi K. K. (2005) Plant Cell Environ. 28, 1037–1045
-
- Horton P., Ruban A. V., Walters R. G. (1996) Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 655–684 - PubMed
-
- Holt N. E., Fleming G. R., Niyogi K. K. (2004) Biochemistry 43, 8281–8289 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

