Activation of phosphatidylinositol 3-kinase/Akt and impairment of nuclear factor-kappaB: molecular mechanisms behind the arrested maturation/activation state of Leishmania infantum-infected dendritic cells
- PMID: 21037075
- PMCID: PMC2993270
- DOI: 10.2353/ajpath.2010.100367
Activation of phosphatidylinositol 3-kinase/Akt and impairment of nuclear factor-kappaB: molecular mechanisms behind the arrested maturation/activation state of Leishmania infantum-infected dendritic cells
Abstract
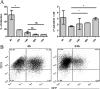
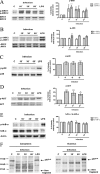
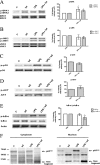
Understanding the complex interactions between Leishmania and dendritic cells (DCs) is central to the modulation of the outcome of this infection, given that an effective immune response against Leishmania is dependent on the successful activation and maturation of DCs. We report here that Leishmania infantum promastigotes successfully infect mouse bone marrow-derived DCs without triggering maturation, as shown by a failure in the up-regulation of CD40 and CD86 expression, and that parasites strongly counteract the lipopolysaccharide-triggered maturation of DCs. A small increase in interleukin (IL)-12 and IL-10 transcription and secretion and a decrease in IL-6 were observed in infected cells. This arrested DC maturation state is actively promoted by parasites because heat-killed or fixed parasites increased cytokine and costimulatory molecule expression. At a molecular level, L. infantum rapidly induced activation of phosphatidylinositol 3-kinase/Akt and extracellular signal-regulated kinase 1/2, whereas no effect was observed in the c-Jun N-terminal kinase and p38 mitogen-activated protein kinase proinflammatory pathways. Moreover, parasites actively promoted cleavage of the nuclear factor-κB p65(RelA) subunit, causing its impairment. The blockade of phosphatidylinositol 3-kinase/Akt by either treatment of bone marrow-derived DCs with wortmannin or transfection with an Akt dominant-negative mutant resulted in a strong decrease in infection rates, revealing for the first time a crucial role of this pathway on Leishmania engulfment by DCs. Overall, our data indicate that activation of Akt and impairment of nuclear factor-κB are responsible for immunogenicity subversion of L. infantum-infected DCs.
Figures




References
-
- Kaye PM, Svensson M, Ato M, Maroof A, Polley R, Stager S, Zubairi S, Engwerda CR. The immunopathology of experimental visceral leishmaniasis. Immunol Rev. 2004;201:239–253. - PubMed
-
- Ghalib HW, Whittle JA, Kubin M, Hashim FA, el-Hassan AM, Grabstein KH, Trinchieri G, Reed SG. IL-12 enhances Th1-type responses in human Leishmania donovani infections. J Immunol. 1995;154:4623–4629. - PubMed
-
- Charmoy M, Megnekou R, Allenbach C, Zweifel C, Perez C, Monnat K, Breton M, Ronet C, Launois P, Tacchini-Cottier F. Leishmania major induces distinct neutrophil phenotypes in mice that are resistant or susceptible to infection. J Leukoc Biol. 2007;82:288–299. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

