Commensal-epithelial signaling mediated via formyl peptide receptors
- PMID: 21037077
- PMCID: PMC2993286
- DOI: 10.2353/ajpath.2010.100529
Commensal-epithelial signaling mediated via formyl peptide receptors
Abstract
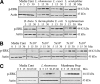
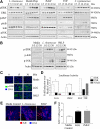
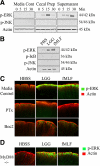
Commensal bacteria and/or their products engender beneficial effects to the mammalian gut, including stimulating physiological cellular turnover and enhancing wound healing, without activating overt inflammation. In the present study, we observed commensal bacteria-mediated activation of the noninflammatory extracellular signal-regulated kinase[ERK]/mitogen-activated protein kinase and Akt signaling pathways in gut epithelial cells and delineated a mechanism for this bacterially activated signaling. All tested strains of commensal bacteria induced ERK phosphorylation without stimulating pro-inflammatory phospho-IκB or pro-apoptotic phospho-c-Jun NH(2)-terminal kinase, with Lactobacillus species being most potent. This pattern of signaling activation was recapitulated using the peptide N-formyl-Met-Leu-Phe, a bacterial product known to stimulate signaling events in mammalian phagocytes. Sensing of N-formyl-Met-Leu-Phe by gut epithelial cells occurs via recently characterized formyl peptide receptors located in the plasma membrane. Both commensal bacteria and N-formyl-Met-Leu-Phe application to the apical surface of polarized gut epithelial cells resulted in specific formyl peptide receptor activation. In addition, pretreatment of model epithelia and murine colon with Boc2 (a specific peptide antagonist) or pertussis toxin (a G(i)-protein inhibitor) abolished commensal-mediated ERK phosphorylation. Taken together, these data show that commensal bacteria specifically activate the ERK/mitogen-activated protein kinase pathway in an formyl peptide receptor-dependent manner, delineating a mechanism by which commensal bacteria contribute to cellular signaling in gut epithelia.
Figures


Similar articles
-
Enteric commensal bacteria induce extracellular signal-regulated kinase pathway signaling via formyl peptide receptor-dependent redox modulation of dual specific phosphatase 3.J Biol Chem. 2011 Nov 4;286(44):38448-38455. doi: 10.1074/jbc.M111.268938. Epub 2011 Sep 15. J Biol Chem. 2011. PMID: 21921027 Free PMC article.
-
Reactive oxygen production induced by the gut microbiota: pharmacotherapeutic implications.Curr Med Chem. 2012;19(10):1519-29. doi: 10.2174/092986712799828283. Curr Med Chem. 2012. PMID: 22360484 Free PMC article. Review.
-
Redox signaling regulates commensal-mediated mucosal homeostasis and restitution and requires formyl peptide receptor 1.Mucosal Immunol. 2014 May;7(3):645-55. doi: 10.1038/mi.2013.84. Epub 2013 Nov 6. Mucosal Immunol. 2014. PMID: 24192910 Free PMC article.
-
Formyl peptide-receptor like-1 requires lipid raft and extracellular signal-regulated protein kinase to activate inhibitor-kappa B kinase in human U87 astrocytoma cells.J Neurochem. 2007 Nov;103(4):1553-66. doi: 10.1111/j.1471-4159.2007.04876.x. Epub 2007 Aug 28. J Neurochem. 2007. PMID: 17727628
-
Redox signaling mediated by the gut microbiota.Free Radic Biol Med. 2017 Apr;105:41-47. doi: 10.1016/j.freeradbiomed.2016.10.495. Epub 2016 Oct 29. Free Radic Biol Med. 2017. PMID: 27989756 Review.
Cited by
-
Enteric commensal bacteria induce extracellular signal-regulated kinase pathway signaling via formyl peptide receptor-dependent redox modulation of dual specific phosphatase 3.J Biol Chem. 2011 Nov 4;286(44):38448-38455. doi: 10.1074/jbc.M111.268938. Epub 2011 Sep 15. J Biol Chem. 2011. PMID: 21921027 Free PMC article.
-
FAM3D is essential for colon homeostasis and host defense against inflammation associated carcinogenesis.Nat Commun. 2020 Nov 20;11(1):5912. doi: 10.1038/s41467-020-19691-z. Nat Commun. 2020. PMID: 33219235 Free PMC article.
-
Reactive oxygen production induced by the gut microbiota: pharmacotherapeutic implications.Curr Med Chem. 2012;19(10):1519-29. doi: 10.2174/092986712799828283. Curr Med Chem. 2012. PMID: 22360484 Free PMC article. Review.
-
Intestinal Microbiota in Colorectal Cancer Surgery.Cancers (Basel). 2020 Oct 16;12(10):3011. doi: 10.3390/cancers12103011. Cancers (Basel). 2020. PMID: 33081401 Free PMC article. Review.
-
Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics.J Clin Invest. 2016 Jun 1;126(6):2049-63. doi: 10.1172/JCI86062. Epub 2016 Apr 25. J Clin Invest. 2016. PMID: 27111232 Free PMC article.
References
-
- Xu J, Mahowald MA, Ley RE, Lozupone CA, Hamady M, Martens EC, Henrissat B, Coutinho PM, Minx P, Latreille P, Cordum H, Van Brunt A, Kim K, Fulton RS, Fulton LA, Clifton SW, Wilson RK, Knight RD, Gordon JI. Evolution of symbiotic bacteria in the distal human intestine. PLoS Biol. 2007;5:e156. - PMC - PubMed
-
- Hooper LV, Bry L, Falk PG, Gordon JI. Host-microbial symbiosis in the mammalian intestine: exploring an internal ecosystem. Bioessays. 1998;20:336–343. - PubMed
-
- Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

