Glatiramer acetate attenuates pro-inflammatory T cell responses but does not directly protect neurons from inflammatory cell death
- PMID: 21037084
- PMCID: PMC2993293
- DOI: 10.2353/ajpath.2010.100442
Glatiramer acetate attenuates pro-inflammatory T cell responses but does not directly protect neurons from inflammatory cell death
Abstract
Glatiramer acetate (GA) is a synthetic, random, basic copolymer capable of modulating adaptive T cell responses. In animal models of various inflammatory and degenerative central nervous system disorders, GA-induced T cells cross the blood-brain barrier, secrete high levels of anti-inflammatory cytokines and neurotrophins, and thus both reduce neuronal damage and promote neurogenesis. Recently, it has been suggested that GA itself may permeate the (impaired) blood-brain-barrier and directly protect neurons under conditions of inflammation-mediated neurodegeneration. To test this hypothesis, we examined the direct effects of GA on neuronal functionality and T cell-mediated neuronal apoptosis in culture, acute brain slices, and focal experimental autoimmune encephalomyelitis. GA caused a depolarization of the resting membrane potential and led to an immediate impairment of action potential generation in neurons. Moreover, GA-incubated neurons underwent dose-dependent apoptosis. Apoptosis of ovalbumin peptide-loaded major histocompatibility complex class I-expressing neurons induced by ovalbumin-specific effector T cells could be reduced by pre-incubation of T cells, but not neurons with GA. Similar results could be found using acute brain slices. In focal experimental autoimmune encephalomyelitis, lesion size and neuronal apoptosis could be limited by pretreating rats with GA, whereas intracerebral GA application into the inflammatory lesion had no effect on neuronal survival. Our data suggest that GA attenuates adaptive pro-inflammatory T cell responses, but does not exert direct neuroprotective effects.
Figures

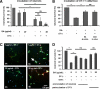
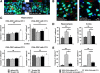

References
-
- Fridkis-Hareli M, Teitelbaum D, Gurevich E, Pecht I, Brautbar C, Kwon OJ, Brenner T, Arnon R, Sela M. Direct binding of myelin basic protein and synthetic copolymer 1 to class II major histocompatibility complex molecules on living antigen-presenting cells–specificity and promiscuity. Proc Natl Acad Sci USA. 1994;91:4872–4876. - PMC - PubMed
-
- Neuhaus O, Farina C, Yassouridis A, Wiendl H, Then Bergh F, Dose T, Wekerle H, Hohlfeld R. Multiple sclerosis: comparison of copolymer-1- reactive T cell lines from treated and untreated subjects reveals cytokine shift from T helper 1 to T helper 2 cells. Proc Natl Acad Sci USA. 2000;97:7452–7457. - PMC - PubMed
-
- Weber MS, Prod'homme T, Youssef S, Dunn SE, Rundle CD, Lee L, Patarroyo JC, Stuve O, Sobel RA, Steinman L, Zamvil SS. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nat Med. 2007;13:935–943. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

