TIM-4, a receptor for phosphatidylserine, controls adaptive immunity by regulating the removal of antigen-specific T cells
- PMID: 21037090
- PMCID: PMC3153437
- DOI: 10.4049/jimmunol.1001360
TIM-4, a receptor for phosphatidylserine, controls adaptive immunity by regulating the removal of antigen-specific T cells
Abstract
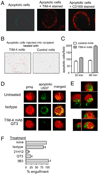
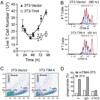
Adaptive immunity is characterized by the expansion of an Ag-specific T cell population following Ag exposure. The precise mechanisms, however, that control the expansion and subsequent contraction in the number of Ag-specific T cells are not fully understood. We show that T cell/transmembrane, Ig, and mucin (TIM)-4, a receptor for phosphatidylserine, a marker of apoptotic cells, regulates adaptive immunity in part by mediating the removal of Ag-specific T cells during the contraction phase of the response. During Ag immunization or during infection with influenza A virus, blockade of TIM-4 on APCs increased the expansion of Ag-specific T cells, resulting in an increase in secondary immune responses. Conversely, overexpression of TIM-4 on APCs in transgenic mice reduced the number of Ag-specific T cells that remained after immunization, resulting in reduced secondary T cell responses. There was no change in the total number of cell divisions that T cells completed, no change in the per cell proliferative capacity of the remaining Ag-specific T cells, and no increase in the development of Ag-specific regulatory T cells in TIM-4 transgenic mice. Thus, TIM-4-expressing cells regulate adaptive immunity by mediating the removal of phosphatidylserine-expressing apoptotic, Ag-specific T cells, thereby controlling the number of Ag-specific T cells that remain after the clearance of Ag or infection.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures




References
-
- Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat. Rev. Immunol. 2007;7:964–974. - PubMed
-
- Borisenko GG, Matsura T, Liu SX, Tyurin VA, Jianfei J, Serinkan FB, Kagan VE. Macrophage recognition of externalized phosphatidylserine and phagocytosis of apoptotic Jurkat cells—existence of a threshold. Arch. Biochem. Biophys. 2003;413:41–52. - PubMed
-
- McIntire JJ, Umetsu SE, Akbari O, Potter M, Kuchroo VK, Barsh GS, Freeman GJ, Umetsu DT, DeKruyff RH. Identification of Tapr (an airway hyperreactivity regulatory locus) and the linked Tim gene family. Nat. Immunol. 2001;2:1109–1116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

