IL-17 regulates adipogenesis, glucose homeostasis, and obesity
- PMID: 21037091
- PMCID: PMC3001125
- DOI: 10.4049/jimmunol.1001269
IL-17 regulates adipogenesis, glucose homeostasis, and obesity
Erratum in
- J Immunol. 2011 Jan 15;186(2):1291
Abstract
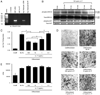
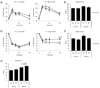
Inflammatory mediators have the potential to impact a surprising range of diseases, including obesity and its associated metabolic syndrome. In this paper, we show that the proinflammatory cytokine IL-17 inhibits adipogenesis, moderates adipose tissue (AT) accumulation, and regulates glucose metabolism in mice. IL-17 deficiency enhances diet-induced obesity in mice and accelerates AT accumulation even in mice fed a low-fat diet. In addition to potential systemic effects, IL-17 is expressed locally in AT by leukocytes, predominantly by γδ T cells. IL-17 suppresses adipocyte differentiation from mouse-derived 3T3-L1 preadipocytes in vitro, and inhibits expression of genes encoding proadipogenic transcription factors, adipokines, and molecules involved in lipid and glucose metabolism. IL-17 also acts on differentiated adipocytes, impairing glucose uptake, and young IL-17-deficient mice show enhanced glucose tolerance and insulin sensitivity. Our findings implicate IL-17 as a negative regulator of adipogenesis and glucose metabolism in mice, and show that it delays the development of obesity.
Conflict of interest statement
B.J.-S. and D.J.C. are employees of MERCK. All other authors have no financial conflicts of interest.
Figures






References
-
- Olufadi R, Byrne CD. Clinical and laboratory diagnosis of the metabolic syndrome. J. Clin. Pathol. 2008;61:697–706. - PubMed
-
- Fajas L. Adipogenesis: a cross-talk between cell proliferation and cell differentiation. Ann. Med. 2003;35:79–85. - PubMed
-
- Fève B. Adipogenesis: cellular and molecular aspects. Best Pract. Res. Clin. Endocrinol. Metab. 2005;19:483–499. - PubMed
-
- Lago F, Dieguez C, Gómez-Reino J, Gualillo O. Adipokines as emerging mediators of immune response and inflammation. Nat. Clin. Pract. Rheumatol. 2007;3:716–724. - PubMed
-
- Goralski KB, McCarthy TC, Hanniman EA, Zabel BA, Butcher EC, Parlee SD, Muruganandan S, Sinal CJ. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J. Biol. Chem. 2007;282:28175–28188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK-084647/DK/NIDDK NIH HHS/United States
- T32 AI007290/AI/NIAID NIH HHS/United States
- F31 AI073198/AI/NIAID NIH HHS/United States
- R37 AI047822/AI/NIAID NIH HHS/United States
- AI-072618/AI/NIAID NIH HHS/United States
- HL-67674/HL/NHLBI NIH HHS/United States
- AI-47822/AI/NIAID NIH HHS/United States
- P50 HL067674/HL/NHLBI NIH HHS/United States
- P30 DK056339/DK/NIDDK NIH HHS/United States
- R01 DK084647/DK/NIDDK NIH HHS/United States
- AI073198/AI/NIAID NIH HHS/United States
- R01 AI047822/AI/NIAID NIH HHS/United States
- AG-028908/AG/NIA NIH HHS/United States
- R01 AI072618/AI/NIAID NIH HHS/United States
- R01 AI079320/AI/NIAID NIH HHS/United States
- DK-56339/DK/NIDDK NIH HHS/United States
- AI-079320/AI/NIAID NIH HHS/United States
- R21 AI047822/AI/NIAID NIH HHS/United States
- P01 HL067674/HL/NHLBI NIH HHS/United States
- R01 AG028908/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

