Autocrine fibroblast growth factor-2 signaling contributes to altered endothelial phenotype in pulmonary hypertension
- PMID: 21037114
- PMCID: PMC4834126
- DOI: 10.1165/rcmb.2010-0317OC
Autocrine fibroblast growth factor-2 signaling contributes to altered endothelial phenotype in pulmonary hypertension
Abstract
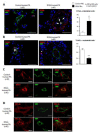
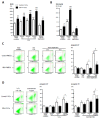
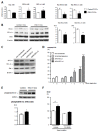
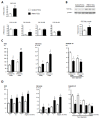
Pulmonary vascular remodeling is key to the pathogenesis of idiopathic pulmonary arterial hypertension (IPAH). We recently reported that fibroblast growth factor (FGF)2 is markedly overproduced by pulmonary endothelial cells (P-ECs) in IPAH and contributes significantly to smooth muscle hyperplasia and disease progression. Excessive FGF2 expression in malignancy exerts pathologic effects on tumor cells by paracrine and autocrine mechanisms.We hypothesized that FGF2 overproduction contributes in an autocrine manner to the abnormal phenotype of P-ECs, characteristic of IPAH. In distal pulmonary arteries (PAs) of patients with IPAH, we found increased numbers of proliferating ECs and decreased numbers of apoptotic ECs, accompanied with stronger immunoreactivity for the antiapoptotic molecules, B-cell lymphoma (BCL)2, and BCL extra long (BCL-xL) compared with PAs from control patients. These in situ observations were replicated in vitro, with cultured P-ECs from patients IPAH exhibiting increased proliferation and diminished sensitivity to apoptotic induction with marked increases in the antiapoptotic factors BCL2 and BCL-xL and levels of phosphorylated extracellular signal-regulated (ERK)1/2 compared with control P-ECs. IPAH P-ECs also exhibited increased FGF2 expression and an accentuated proliferative and survival response to conditioned P-EC media or exogenous FGF2 treatment. Decreasing FGF2 signaling by RNA interference normalized sensitivity to apoptosis and proliferative potential in the IPAH P-ECs. Our findings suggest that excessive autocrine release of endothelial-derived FGF2 in IPAH contributes to the acquisition and maintenance of an abnormal EC phenotype, enhancing proliferation through constitutive activation of ERK1/2 and decreasing apoptosis by increasing BCL2 and BCL-xL.
Conflict of interest statement
None.
Figures
Similar articles
-
Endothelial-derived FGF2 contributes to the progression of pulmonary hypertension in humans and rodents.J Clin Invest. 2009 Mar;119(3):512-23. doi: 10.1172/JCI35070. Epub 2009 Feb 9. J Clin Invest. 2009. PMID: 19197140 Free PMC article.
-
Key role of the endothelial TGF-β/ALK1/endoglin signaling pathway in humans and rodents pulmonary hypertension.PLoS One. 2014 Jun 23;9(6):e100310. doi: 10.1371/journal.pone.0100310. eCollection 2014. PLoS One. 2014. PMID: 24956016 Free PMC article.
-
Role of endothelium-derived CC chemokine ligand 2 in idiopathic pulmonary arterial hypertension.Am J Respir Crit Care Med. 2007 Nov 15;176(10):1041-7. doi: 10.1164/rccm.200610-1559OC. Epub 2007 Sep 6. Am J Respir Crit Care Med. 2007. PMID: 17823354
-
[Control of the intracellular signaling induced by fibroblast growth factors (FGF) over the proliferation and survival of retinal pigment epithelium cells: example of the signaling regulation of growth factors endogenous to the retina ].J Soc Biol. 2001;195(2):101-6. J Soc Biol. 2001. PMID: 11723820 Review. French.
-
Mechanisms of disease: pulmonary arterial hypertension.Nat Rev Cardiol. 2011 Jun 21;8(8):443-55. doi: 10.1038/nrcardio.2011.87. Nat Rev Cardiol. 2011. PMID: 21691314 Free PMC article. Review.
Cited by
-
Dysregulated renin-angiotensin-aldosterone system contributes to pulmonary arterial hypertension.Am J Respir Crit Care Med. 2012 Oct 15;186(8):780-9. doi: 10.1164/rccm.201203-0411OC. Epub 2012 Aug 2. Am J Respir Crit Care Med. 2012. PMID: 22859525 Free PMC article.
-
Dasatinib induces lung vascular toxicity and predisposes to pulmonary hypertension.J Clin Invest. 2016 Sep 1;126(9):3207-18. doi: 10.1172/JCI86249. Epub 2016 Aug 2. J Clin Invest. 2016. PMID: 27482885 Free PMC article. Clinical Trial.
-
An endothelial apelin-FGF link mediated by miR-424 and miR-503 is disrupted in pulmonary arterial hypertension.Nat Med. 2013 Jan;19(1):74-82. doi: 10.1038/nm.3040. Epub 2012 Dec 23. Nat Med. 2013. PMID: 23263626 Free PMC article.
-
Endothelial cells in the pathogenesis of pulmonary arterial hypertension.Eur Respir J. 2021 Sep 2;58(3):2003957. doi: 10.1183/13993003.03957-2020. Print 2021 Sep. Eur Respir J. 2021. PMID: 33509961 Free PMC article. Review.
-
Bone morphogenetic protein-9 controls pulmonary vascular growth and remodeling.Proc Natl Acad Sci U S A. 2025 Jul;122(26):e2410229122. doi: 10.1073/pnas.2410229122. Epub 2025 Jun 23. Proc Natl Acad Sci U S A. 2025. PMID: 40549904
References
-
- Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, Gomez-Sanchez MA, Jondeau G, Klepetko W, Opitz C, Peacock A, Rubin L, Zellweger M, Simonneau G. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219–1263. - PubMed
-
- Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin MT, Jing ZC, Krowka MJ, Langleben D, Nakanishi N, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43–54. - PubMed
-
- Hassoun PM, Mouthon L, Barbera JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, Newman JH, Rabinovitch M, Schermuly R, Stenmark KR, Voelkel NF, Yuan JX, Humbert M. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;54:S10–19. - PubMed
-
- Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:13S–24S. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

