The role of the prokineticin 2 pathway in human reproduction: evidence from the study of human and murine gene mutations
- PMID: 21037178
- PMCID: PMC3365793
- DOI: 10.1210/er.2010-0007
The role of the prokineticin 2 pathway in human reproduction: evidence from the study of human and murine gene mutations
Abstract
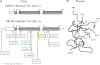
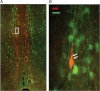
A widely dispersed network of hypothalamic GnRH neurons controls the reproductive axis in mammals. Genetic investigation of the human disease model of isolated GnRH deficiency has revealed several key genes crucial for GnRH neuronal ontogeny and GnRH secretion. Among these genes, prokineticin 2 (PROK2), and PROK2 receptor (PROKR2) have recently emerged as critical regulators of reproduction in both mice and humans. Both prok2- and prokr2-deficient mice recapitulate the human Kallmann syndrome phenotype. Additionally, PROK2 and PROKR2 mutations are seen in humans with Kallmann syndrome, thus implicating this pathway in GnRH neuronal migration. However, PROK2/PROKR2 mutations are also seen in normosmic GnRH deficiency, suggesting a role for the prokineticin signaling system in GnRH biology that is beyond neuronal migration. This observation is particularly surprising because mature GnRH neurons do not express PROKR2. Moreover, mutations in both PROK2 and PROKR2 are predominantly detected in the heterozygous state with incomplete penetrance or variable expressivity frequently seen within and across pedigrees. In some of these pedigrees, a "second hit" or oligogenicity has been documented. Besides reproduction, a pleiotropic physiological role for PROK2 is now recognized, including regulation of pain perception, circadian rhythms, hematopoiesis, and immune response. Therefore, further detailed clinical studies of patients with PROK2/PROKR2 mutations will help to map the broader biological role of the PROK2/PROKR2 pathway and identify other interacting genes/proteins that mediate its molecular effects in humans.
Figures


References
-
- Crowley WF, Jr, Jameson JL. 1992. Clinical counterpoint: gonadotropin-releasing hormone deficiency: perspectives from clinical investigation. Endocr Rev 13:635–640 - PubMed
-
- Merchenthaler I, Görcs T, Sétáló G, Petrusz P, Flerkó B. 1984. Gonadotropin-releasing hormone (GnRH) neurons and pathways in the rat brain. Cell Tissue Res 237:15–29 - PubMed
-
- Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E. 1978. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science 202:631–633 - PubMed
-
- Waldhauser F, Weissenbacher G, Frisch H, Pollak A. 1981. Pulsatile secretion of gonadotropins in early infancy. Eur J Pediatr 137:71–74 - PubMed
-
- Wu FC, Butler GE, Kelnar CJ, Sellar RE. 1990. Patterns of pulsatile luteinizing hormone secretion before and during the onset of puberty in boys: a study using an immunoradiometric assay. J Clin Endocrinol Metab 70:629–637 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

