Hypotonic swelling-induced activation of PKN1 mediates cell survival in cardiac myocytes
- PMID: 21037231
- PMCID: PMC3023260
- DOI: 10.1152/ajpheart.00232.2010
Hypotonic swelling-induced activation of PKN1 mediates cell survival in cardiac myocytes
Abstract
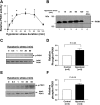
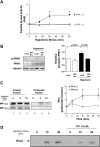
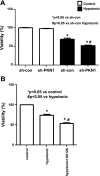
Hypotonic cell swelling in the myocardium is induced by pathological conditions, including ischemia-reperfusion, and affects the activities of ion transporters/channels and gene expression. However, the signaling mechanism activated by hypotonic stress (HS) is not fully understood in cardiac myocytes. A specialized protein kinase cascade, consisting of Pkc1 and MAPKs, is activated by HS in yeast. Here, we demonstrate that protein kinase N1 (PKN1), a serine/threonine protein kinase and a homolog of Pkc1, is activated by HS (67% osmolarity) within 5 min and reaches peak activity at 60 min in cardiac myocytes. Activation of PKN1 by HS was accompanied by Thr(774) phosphorylation and concomitant activation of PDK1, a potential upstream regulator of PKN1. HS also activated RhoA, thereby increasing interactions between PKN1 and RhoA. PP1 (10(-5) M), a selective Src family tyrosine kinase inhibitor, significantly suppressed HS-induced activation of RhoA and PKN1. Constitutively active PKN1 significantly increased the transcriptional activity of Elk1-GAL4, an effect that was inhibited by dominant negative MEK. Overexpression of PKN1 significantly increased ERK phosphorylation, whereas downregulation of PKN1 inhibited HS-induced ERK phosphorylation. Downregulation of PKN1 and inhibition of ERK by U-0126 both significantly inhibited the survival of cardiac myocytes in the presence of HS. These results suggest that a signaling cascade, consisting of Src, RhoA, PKN1, and ERK, is activated by HS, thereby promoting cardiac myocyte survival.
Figures



Similar articles
-
Protein Kinase N Promotes Stress-Induced Cardiac Dysfunction Through Phosphorylation of Myocardin-Related Transcription Factor A and Disruption of Its Interaction With Actin.Circulation. 2019 Nov 19;140(21):1737-1752. doi: 10.1161/CIRCULATIONAHA.119.041019. Epub 2019 Sep 30. Circulation. 2019. PMID: 31564129
-
Tyrosine kinase activation is an immediate and essential step in hypotonic cell swelling-induced ERK activation and c-fos gene expression in cardiac myocytes.EMBO J. 1996 Oct 15;15(20):5535-46. EMBO J. 1996. PMID: 8896447 Free PMC article.
-
c-Raf/MEK/ERK pathway controls protein kinase C-mediated p70S6K activation in adult cardiac muscle cells.J Biol Chem. 2002 Jun 21;277(25):23065-75. doi: 10.1074/jbc.M200328200. Epub 2002 Apr 8. J Biol Chem. 2002. PMID: 11940578
-
Oxidative stress activates extracellular signal-regulated kinases through Src and Ras in cultured cardiac myocytes of neonatal rats.J Clin Invest. 1997 Oct 1;100(7):1813-21. doi: 10.1172/JCI119709. J Clin Invest. 1997. PMID: 9312182 Free PMC article.
-
Cell type-specific angiotensin II-evoked signal transduction pathways: critical roles of Gbetagamma subunit, Src family, and Ras in cardiac fibroblasts.Circ Res. 1998 Feb 23;82(3):337-45. doi: 10.1161/01.res.82.3.337. Circ Res. 1998. PMID: 9486662 Review.
Cited by
-
Loss of Protein Kinase Novel 1 (PKN1) is associated with mild systolic and diastolic contractile dysfunction, increased phospholamban Thr17 phosphorylation, and exacerbated ischaemia-reperfusion injury.Cardiovasc Res. 2018 Jan 1;114(1):138-157. doi: 10.1093/cvr/cvx206. Cardiovasc Res. 2018. PMID: 29045568 Free PMC article.
-
Increase in hypotonic stress-induced endocytic activity in macrophages via ClC-3.Mol Cells. 2014 May;37(5):418-25. doi: 10.14348/molcells.2014.0031. Epub 2014 May 16. Mol Cells. 2014. PMID: 24850147 Free PMC article.
-
Verteporfin treatment controls morphology, phenotype, and global gene expression for cells of the human nucleus pulposus.JOR Spine. 2020 Jul 30;3(4):e1111. doi: 10.1002/jsp2.1111. eCollection 2020 Dec. JOR Spine. 2020. PMID: 33392449 Free PMC article.
-
PKN1 kinase-negative knock-in mice develop splenomegaly and leukopenia at advanced age without obvious autoimmune-like phenotypes.Sci Rep. 2019 Sep 27;9(1):13977. doi: 10.1038/s41598-019-50419-2. Sci Rep. 2019. PMID: 31562379 Free PMC article.
-
Regulation of epithelial sodium transport via epithelial Na+ channel.J Biomed Biotechnol. 2011;2011:978196. doi: 10.1155/2011/978196. Epub 2011 Oct 17. J Biomed Biotechnol. 2011. PMID: 22028593 Free PMC article. Review.
References
-
- Aikawa R, Komuro I, Yamazaki T, Zou Y, Kudoh S, Zhu W, Kadowaki T, Yazaki Y. Rho family small G proteins play critical roles in mechanical stress- induced hypertrophic responses in cardiac myocytes. Circ Res 84: 458–466, 1999 - PubMed
-
- Amano M, Mukai H, Ono Y, Chihara K, Matsui T, Hamajima Y, Okawa K, Iwamatsu A, Kaibuchi K. Identification of a putative target for Rho as the serine-threonine kinase protein kinase N. Science 271: 648–650, 1996 - PubMed
-
- Aoki H, Izumo S, Sadoshima J. Angiotensin II activates RhoA in cardiac myocytes:a critical role of RhoA in angiotensin II-induced premyofibril formation. Circ Res 81: 666–676, 1998 - PubMed
-
- Armstrong SC, Shivell LC, Ganote CE. Sarcolemmal blebs and osmotic fragility as correlates of irreversible ischemic injury in preconditioned isolated rabbit cardiomyocytes. J Mol Cell Cardiol 33: 149–160, 2001 - PubMed
-
- Atienza JM, Suh M, Xenarios I, Landgraf R, Colicelli J. Human ERK1 induces filamentous growth and cell wall remodeling pathways in Saccharomyces cerevisiae. J Biol Chem 275: 20638–20646, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

