Expanding the chemical scope of RNA:methyltransferases to site-specific alkynylation of RNA for click labeling
- PMID: 21037259
- PMCID: PMC3061074
- DOI: 10.1093/nar/gkq825
Expanding the chemical scope of RNA:methyltransferases to site-specific alkynylation of RNA for click labeling
Abstract
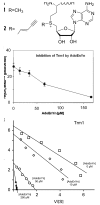
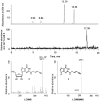
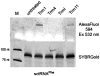
This work identifies the combination of enzymatic transfer and click labeling as an efficient method for the site-specific tagging of RNA molecules for biophysical studies. A double-activated analog of the ubiquitous co-substrate S-adenosyl-l-methionine was employed to enzymatically transfer a five carbon chain containing a terminal alkynyl moiety onto RNA. The tRNA:methyltransferase Trm1 transferred the extended alkynyl moiety to its natural target, the N2 of guanosine 26 in tRNA(Phe). LC/MS and LC/MS/MS techniques were used to detect and characterize the modified nucleoside as well as its cycloaddition product with a fluorescent azide. The latter resulted from a labeling reaction via Cu(I)-catalyzed azide-alkyne 1,3-cycloaddition click chemistry, producing site-specifically labeled RNA whose suitability for single molecule fluorescence experiments was verified in fluorescence correlation spectroscopy experiments.
Figures



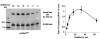

References
-
- Bujnicki JM, Droogmans L, Grosjean H, Purushothaman SK, Lapeyre B. Bioinformatics-guided identification and experimental characterization of novel RNA methyltransferases. Nucleic Acids Mol. Biol. 2004;15:139–168.
-
- Michelot R, Legreverend M, Farrugia G, Lederer E. New studies on inhibition of tRNA N2 guanine methyltransferase by S-adenosyl-homocysteine and S-adenosyl-methionine analogs. Biochimie. 1976;58:201–205. - PubMed
-
- Leboy PS, Glick JM, Steiner FG, Haney S, Borchardt RT. S-adenosylhomocysteine analogues as inhibitors of specific tRNA methylation. Biochim. Biophys. Acta. 1978;520:153–163. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

