Biopharmaceuticals and monoclonal antibodies in oncology trials--a cross-sectional analysis
- PMID: 21037277
- PMCID: PMC3003447
- DOI: 10.1093/protein/gzq090
Biopharmaceuticals and monoclonal antibodies in oncology trials--a cross-sectional analysis
Abstract
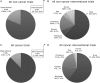
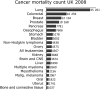
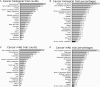
Protein engineering has led to a significantly improved understanding of the biophysical properties of proteins and, importantly, of the molecular mechanisms of disease. Moreover, it has enabled scientists to modify the molecular characteristics of peptides and proteins, leading to improved pharmacokinetics and pharmacodynamics of protein therapeutics. Consequently, biopharmaceuticals, such as monoclonal antibodies (mAbs), interferons/cytokines or vaccines, contribute increasingly to clinical practice. Some of these new treatments have dramatically changed the outcome of specific diseases. However, treatment options remain limited in many conditions, particularly in malignant disease, despite a much-improved understanding of the molecular mechanisms underlying cancer. With the successful pre-clinical development of therapeutic biomolecules, the most significant barrier prior to implementation into clinical practice is proof of concept in humans. This is in part addressed by clinical trials that evaluate the toxicology, dose response and efficacy of the molecules. This observational study summarises the current state of biopharmaceuticals in clinical trials and provides a particular focus on oncology trials. It identifies those cancer types that are most likely to benefit from the efforts made in pre-clinical protein science and establishes evidence that engineered proteins and peptides are set to play a growing role in clinical practice. This study was based on the 95,254 trials registered on the National Institute of Health Clinical Trials Database by 31 August 2010. Of these, 25,525 trials assigned to cancer conditions, including leukaemia and lymphoma, were further analysed, with a particular focus on the 3653 interventional trials that were based on biological interventions. The inclusion criterion for the analysis was registration on the Clinical Trials Database by the above date. No other trials were included. Biopharmaceuticals were the more prevalent intervention in cancer trials (14%) compared with trials in non-cancer conditions (6%). Further subgroup analysis based on the 20 cancer subtypes with the highest mortality revealed that biological therapeutics comprise 43% in malignant melanoma trials and more than 20% in five other cancer types. Two-thirds of all monoclonal antibody are registered in cancer trials (1033, 4.6% of all cancer trials). The subgroup analysis demonstrated a predominance of lymphoma and leukaemia trials for antibody interventions, with 204 and 163 trials registered, respectively. In non-cancer conditions only 503 (0.9%) trials investigate monoclonal antibody interventions. A retrospective longitudinal analysis of the trials demonstrated that monoclonal antibody trials are increasingly frequently registered in non-cancer as well as cancer conditions. However, biopharmaceutical trials continue to be registered more frequently only in non-cancer conditions, but have come to a plateau in cancers. This study is limited by analysis of data from one database only. While the NIH Clinical Trials Database used is the most comprehensive and internationally recognised of its kind, it is possible that the results may have been modified if other databases were also included. Protein engineering has paved the way for biopharmaceutical clinical interventions. A cross-sectional analysis of trials registered on the NIH Clinical Trial Database shows that biological interventions are increasingly entered into clinical trials. While oncological diseases used to lead this effort, biotherapeutic trials in non-cancer conditions have now become more frequent in comparison. Monoclonal antibodies, however, are still mainly investigated in oncological conditions. Haemato-oncological diseases are most frequently investigated for mAb interventions, although they are not among the eight most common causes of cancer mortality. This may reflect the fact that pre-clinical research, understanding of molecular mechanisms and target identification in other malignancies and diseases is less developed.
Figures
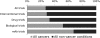
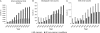
Similar articles
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Epidermal growth factor receptor inhibition strategies in oncology.Endocr Relat Cancer. 2004 Dec;11(4):689-708. doi: 10.1677/erc.1.00600. Endocr Relat Cancer. 2004. PMID: 15613446 Review.
-
Oblimersen: Augmerosen, BCL-2 antisense oligonucleotide - Genta, G 3139, GC 3139, oblimersen sodium.Drugs R D. 2007;8(5):321-34. doi: 10.2165/00126839-200708050-00006. Drugs R D. 2007. PMID: 17767397 Review.
-
Monoclonal antibodies in clinical oncology.Anticancer Agents Med Chem. 2008 Jun;8(5):523-32. doi: 10.2174/187152008784533071. Anticancer Agents Med Chem. 2008. PMID: 18537534 Review.
-
Phage Display Derived Monoclonal Antibodies: From Bench to Bedside.Front Immunol. 2020 Aug 28;11:1986. doi: 10.3389/fimmu.2020.01986. eCollection 2020. Front Immunol. 2020. PMID: 32983137 Free PMC article. Review.
Cited by
-
Cross-sectional analysis of data from the U.S. clinical trials database reveals poor translational clinical trial effort for traumatic brain injury, compared with stroke.PLoS One. 2014 Jan 8;9(1):e84336. doi: 10.1371/journal.pone.0084336. eCollection 2014. PLoS One. 2014. PMID: 24416218 Free PMC article.
-
Cross-sectional and longitudinal analysis of cancer vaccination trials registered on the US Clinical Trials Database demonstrates paucity of immunological trial endpoints and decline in registration since 2008.Drug Des Devel Ther. 2014 Sep 27;8:1539-53. doi: 10.2147/DDDT.S65963. eCollection 2014. Drug Des Devel Ther. 2014. PMID: 25302014 Free PMC article.
References
-
- Clinical Trials Database ‘US National Institute of Health Trial Registry’. http://clinicaltrials.gov/ doi:10.1007/BF02672073. - DOI
-
- Friedler A., DeDecker B.S., Freund S.M., Blair C., Rudiger S., Fersht A.R. J. Mol. Biol. 2004;336:187–196. doi:10.1007/BF00935980. - DOI - PubMed
-
- Heinis C., Rutherford T., Freund S., Winter G. Nat. Chem. Biol. 2009;5:502–507. doi:10.1139/g95-128. - DOI - PubMed
-
- Holt L.J., Herring C., Jespers L.S., Woolven B.P., Tomlinson I.M. Trends Biotechnol. 2003;21:484–490. - PubMed
-
- Hughes B. Nat. Rev. Drug Discov. 2009;9:89–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

