Nuclear targeting of a bacterial integrase that mediates site-specific recombination between bacterial and human target sequences
- PMID: 21037296
- PMCID: PMC3019720
- DOI: 10.1128/AEM.01371-10
Nuclear targeting of a bacterial integrase that mediates site-specific recombination between bacterial and human target sequences
Abstract
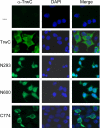
TrwC is a bacterial protein involved in conjugative transfer of plasmid R388. It is transferred together with the DNA strand into the recipient bacterial cell, where it can integrate the conjugatively transferred DNA strand into its target sequence present in the recipient cell. Considering that bacterial conjugation can occur between bacteria and eukaryotic cells, this protein has great biotechnological potential as a site-specific integrase. We have searched for possible TrwC target sequences in the human genome. Recombination assays showed that TrwC efficiently catalyzes recombination between its natural target sequence and a discrete number of sequences, located in noncoding sites of the human genome, which resemble this target. We have determined the cellular localization of TrwC and derivatives in human cells by immunofluorescence and also by an indirect yeast-based assay to detect both nuclear import and export signals. The results indicate that the recombinase domain of TrwC (N600) has nuclear localization, but full-length TrwC locates in the cytoplasm, apparently due to the presence of a nuclear export signal in its C-terminal domain. The recombinase domain of TrwC can be transported to recipient cells by conjugation in the presence of the helicase domain of TrwC, but with very low efficiency. We mutagenized the trwC gene and selected for mutants with nuclear localization. We obtained one such mutant with a point A904T mutation and an extra peptide at its C terminus, which maintained its functionality in conjugation and recombination. This TrwC mutant could be useful for future TrwC-mediated site-specific integration assays in mammalian cells.
Figures

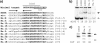



References
-
- Bartolomé, B., Y. Jubete, E. Martínez, and F. de la Cruz. 1991. Construction and properties of a family of pACYC184-derived cloning vectors compatible with pBR322 and its derivatives. Gene 102:75-78. - PubMed
-
- Buchanan-Wollaston, V., J. E. Passiatore, and F. Cannon. 1987. The mob and oriT mobilization functions of a bacterial plasmid promote its transfer to plants. Nature 328:172-175.
-
- Cambronne, E. D., and C. R. Roy. 2006. Recognition and delivery of effector proteins into eukaryotic cells by bacterial secretion systems. Traffic 7:929-939. - PubMed

