Functional Models for the Oxygen-Evolving Complex of Photosystem II
- PMID: 21037800
- PMCID: PMC2966027
- DOI: 10.1016/j.ccr.2007.06.002
Functional Models for the Oxygen-Evolving Complex of Photosystem II
Abstract
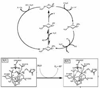
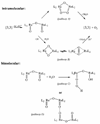
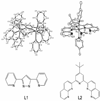
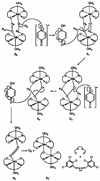
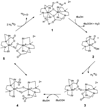
In the last ten years, a number of advances have been made in the study of the oxygen-evolving complex (OEC) of photosystem II (PSII). Along with this new understanding of the natural system has come rapid advance in chemical models of this system. The advance of PSII model chemistry is seen most strikingly in the area of functional models where the few known systems available when this topic was last reviewed has grown into two families of model systems. In concert with this work, numerous mechanistic proposals for photosynthetic water oxidation have been proposed. Here, we review the recent efforts in functional model chemistry of the oxygen-evolving complex of photosystem II.
Figures
Similar articles
-
The O2-Evolving Complex of Photosystem II: Recent Insights from Quantum Mechanics/Molecular Mechanics (QM/MM), Extended X-ray Absorption Fine Structure (EXAFS), and Femtosecond X-ray Crystallography Data.Acc Chem Res. 2017 Jan 17;50(1):41-48. doi: 10.1021/acs.accounts.6b00405. Epub 2016 Dec 21. Acc Chem Res. 2017. PMID: 28001034 Review.
-
Relative stability of the S2 isomers of the oxygen evolving complex of photosystem II.Photosynth Res. 2019 Sep;141(3):331-341. doi: 10.1007/s11120-019-00637-6. Epub 2019 Apr 2. Photosynth Res. 2019. PMID: 30941614
-
Structural changes of the oxygen-evolving complex in photosystem II during the catalytic cycle.J Biol Chem. 2013 Aug 2;288(31):22607-20. doi: 10.1074/jbc.M113.476622. Epub 2013 Jun 13. J Biol Chem. 2013. PMID: 23766513 Free PMC article.
-
Structural models of the manganese complex of photosystem II and mechanistic implications.Biochim Biophys Acta. 2012 Jan;1817(1):88-105. doi: 10.1016/j.bbabio.2011.07.004. Epub 2011 Jul 20. Biochim Biophys Acta. 2012. PMID: 21787743
-
Structural Coupling of Extrinsic Proteins with the Oxygen-Evolving Center in Photosystem II.Front Plant Sci. 2016 Feb 5;7:84. doi: 10.3389/fpls.2016.00084. eCollection 2016. Front Plant Sci. 2016. PMID: 26904056 Free PMC article. Review.
Cited by
-
Imidazolium or guanidinium/layered manganese (III, IV) oxide hybrid as a promising structural model for the water-oxidizing complex of Photosystem II for artificial photosynthetic systems.Photosynth Res. 2013 Nov;117(1-3):413-21. doi: 10.1007/s11120-013-9814-5. Epub 2013 Mar 31. Photosynth Res. 2013. PMID: 23543329
-
Proteomic changes induced by potassium deficiency and potassium substitution by sodium in sugar beet.J Plant Res. 2016 May;129(3):527-38. doi: 10.1007/s10265-016-0800-9. Epub 2016 Feb 9. J Plant Res. 2016. PMID: 26860314
-
Highly active and robust Cp* iridium complexes for catalytic water oxidation.J Am Chem Soc. 2009 Jul 1;131(25):8730-1. doi: 10.1021/ja901270f. J Am Chem Soc. 2009. PMID: 19496565 Free PMC article.
-
Energy conversion in natural and artificial photosynthesis.Chem Biol. 2010 May 28;17(5):434-47. doi: 10.1016/j.chembiol.2010.05.005. Chem Biol. 2010. PMID: 20534342 Free PMC article. Review.
-
Exceptional Quantum Efficiency Powers Biomass Production in Halotolerant Algae Picochlorum sp.Photosynth Res. 2024 Dec;162(2-3):439-457. doi: 10.1007/s11120-024-01075-9. Epub 2024 Feb 8. Photosynth Res. 2024. PMID: 38329705
References
-
- Ferreira KN, Iverson TM, Maghlaoui K, Barber J, Iwata S. Science. 2004;303:1831–1838. - PubMed
-
- Loll B, Kern J, Saenger W, Zouni A, Biesadka J. Nature. 2005;438:1040–1044. - PubMed
-
- Haumann M, Müller C, Liebisch P, Iuzzolino L, Dittmer J, Grabolle M, Neisius T, Meyer-Klaucke W, Dau H. Biochemistry. 2005;44:1894–1908. - PubMed
-
- Haumann M, Liebisch P, Müller C, Barra M, Grabolle M, Dau H. Science. 2005;310:1019–1021. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
