Acquisition of regulatory function by human CD8(+) T cells treated with anti-CD3 antibody requires TNF
- PMID: 21038470
- PMCID: PMC3073342
- DOI: 10.1002/eji.201040485
Acquisition of regulatory function by human CD8(+) T cells treated with anti-CD3 antibody requires TNF
Abstract
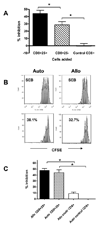
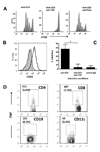
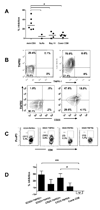
Anti-CD3 mAb can modulate graft rejection and attenuate autoimmune diseases but their mechanism(s) of action remain unclear. CD8(+) T cells with regulatory function are induced in vitro by Teplizumab, a humanized anti-CD3 antibody and inhibit responses of autologous and allogeneic T cells. They inhibit CD4(+) T-cell proliferation by mechanisms involving TNF and CCL4, and by blocking target cell entry into G2/M phase of cell cycle but neither kill them, nor compete for IL-2. CD8(+) Treg can be isolated from peripheral blood following treatment of patients with Type 1 diabetes with Teplizumab, but not from untreated patients. The induction of CD8(+) Treg by anti-CD3 mAb requires TNF and signaling through the NF-κB cascade. The CD8(+) Treg express CD25, glucocorticoid-induced TNF receptor family, CTLA-4, Foxp3, and TNFR2, and the combined expression of TNFR2 and CD25 identifies a potent subpopulation of CD8(+) Treg. These studies have identified a novel mechanism of immune regulation by anti-CD3 mAb and markers that may be used to track inducible CD8(+) Treg in settings such as chronic inflammation or immune therapy.
Conflict of interest statement
The authors declare that they do not have conflicts of interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

