Methaneseleninic acid is a substrate for truncated mammalian thioredoxin reductase: implications for the catalytic mechanism and redox signaling
- PMID: 21038895
- PMCID: PMC3018153
- DOI: 10.1021/bi101130t
Methaneseleninic acid is a substrate for truncated mammalian thioredoxin reductase: implications for the catalytic mechanism and redox signaling
Abstract
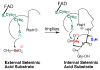
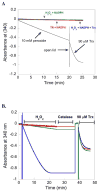
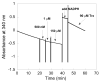
Mammalian thioredoxin reductase is a homodimeric pyridine nucleotide disulfide oxidoreductase that contains the rare amino acid selenocysteine (Sec) on a C-terminal extension. We previously have shown that a truncated version of mouse mitochondrial thioredoxin reductase missing this C-terminal tail will catalyze the reduction of a number of small molecules. Here we show that the truncated thioredoxin reductase will catalyze the reduction of methaneseleninic acid. This reduction is fast at pH 6.1 and is only 4-fold slower than that of the full-length enzyme containing Sec. This finding suggested to us that if the C-terminal Sec residue in the holoenzyme became oxidized to the seleninic acid form (Sec-SeO(2)(-)) that it would be quickly reduced back to an active state by enzymic thiols and further suggested to us that the enzyme would be very resistant to irreversible inactivation by oxidation. We tested this hypothesis by reducing the enzyme with NADPH and subjecting it to high concentrations of H(2)O(2) (up to 50 mM). The results show that the enzyme strongly resisted inactivation by 50 mM H(2)O(2). To determine the redox state of the C-terminal Sec residue, we attempted to inhibit the enzyme with dimedone. Dimedone alkylates protein sulfenic acid residues and presumably will alkylate selenenic acid (Sec-SeOH) residues as well. The enzyme was not inhibited by dimedone even when a 150-fold excess was added to the reaction mixture containing the enzyme and H(2)O(2). We also tested the ability of the truncated enzyme to resist inactivation by oxidation as well and found that it also was resistant to high concentrations of H(2)O(2). One assumption for the use of Sec in enzymes is that it is catalytically superior to the use of cysteine. We and others have previously suggested that there are reasons for the use of Sec in enzymes that are unrelated to the conversion of substrate to product. The data presented here support this assertion. The results also imply that the redox signaling function of the thioredoxin system can remain active under oxidative stress.
Figures



References
-
- Bock A, Forchhammer K, Heider J, Leinfelder W, Sawers G, Veprek B, Zinoni F. Selenocysteine: the 21st amino acid. Mol Microbiol. 1991;5:515–520. - PubMed
-
- Atkins JF, Gesteland RF. The twenty-first amino acid. Nature. 2000;407:463–465. - PubMed
-
- Sun QA, Wu Y, Zappacosta F, Jeang KT, Lee BJ, Hatfield DL, Gladyshev VN. Redox regulation of cell signaling by selenocysteine in mammalian thioredoxin reductases. J Biol Chem. 1999;274:24522–24530. - PubMed
-
- Carugo O, Cemazar M, Zahariev S, Hudáky I, Gáspári Z, Perczel A, Pongor S. Vicinal disulfide turns. Protein Eng. 2003;16:637–639. - PubMed
-
- Hudaky I, Gaspari Z, Carugo O, Cemazar M, Pongor S, Perczel A. Vicinal disulfide bridge conformers by experimental methods and by ab initio and DFT molecular computations. Proteins. 2004;55:152–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

