Pharmacological characterization of six trkB antibodies reveals a novel class of functional agents for the study of the BDNF receptor
- PMID: 21039416
- PMCID: PMC3042204
- DOI: 10.1111/j.1476-5381.2010.01094.x
Pharmacological characterization of six trkB antibodies reveals a novel class of functional agents for the study of the BDNF receptor
Abstract
Background and purpose: By interacting with trkB receptors, brain-derived neurotrophic factor (BDNF) triggers various signalling pathways responsible for neurone survival, differentiation and modulation of synaptic transmission. Numerous reports have implicated BDNF and trkB in the pathogenesis of various central nervous system affections and in cancer, thus representing trkB as a promising therapeutic target. In this study, we used an antibody-based approach to search for trkB-selective functional reagents.
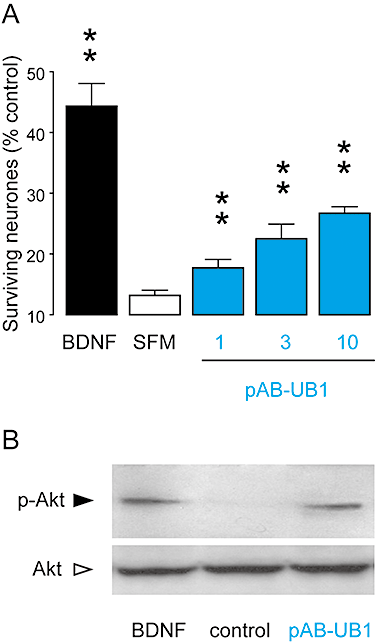
Experimental approach: Six commercially available polyclonal and monoclonal antibodies were tested on recombinant and native, human and rodent trkB receptors. Functional and pharmacological characterization was performed using a modified version of the KIRA-elisa method and radioligand binding studies. Western blot analyses and neurite outgrowth assays were carried out to determine the specificity and selectivity of antibody effects. The survival properties of one antibody were further assessed on cultured neurones in a serum-deprived paradigm.
Key results: The functional trkB-selective antibodies showed distinct pharmacological profiles, ranging from partial agonists to antagonists, acting on trkB receptors through allosteric modulations. The same diversity of effects was observed on the mitogen-activated protein kinase signalling pathway downstream of trkB and on the subsequent neurite outgrowth. One antibody with partial agonist activity demonstrated cell survival properties by activating the Akt pathway. Finally, these antibodies were functionally validated as true trkB-selective ligands because they failed activating trkA or trkC, and contrary to BDNF, none of them bind to p75(NTR).
Conclusions and implications: These trkB-selective antibodies represent a novel class of pharmacological tools to explore the pathophysiological roles of trkB and its potential therapeutic relevance for the treatment of various disorders.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures



References
-
- Banfield MJ, Naylor RL, Robertson AG, Allen SJ, Dawbarn D, Brady RL. Specificity in Trk receptor: neurotrophin interactions: the crystal structure of TrkB-d5 in complex with neurotrophin-4/5. Structure (Camb) 2001;9:1191–1199. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

