Mechanisms by which chronic ethanol feeding limits the ability of dendritic cells to stimulate T-cell proliferation
- PMID: 21039629
- PMCID: PMC3058243
- DOI: 10.1111/j.1530-0277.2010.01321.x
Mechanisms by which chronic ethanol feeding limits the ability of dendritic cells to stimulate T-cell proliferation
Abstract
Background: As initiators of immune responses, dendritic cells (DCs) are required for antigen (Ag)-specific activation of naïve T cells in the defense against infectious agents. The increased susceptibility to and severity of infection seen in chronic alcoholics could be because of impaired DCs initiation of naïve T-cell responses. Specifically, these DCs may not provide adequate Signals 1 (Ag presentation), 2 (costimulation), or 3 (cytokine production) to these T cells.
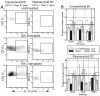
Methods: Using the Meadows-Cook murine model of chronic alcohol abuse, the ability of ethanol (EtOH)-exposed DCs to stimulate T-cell proliferation, acquire and process Ag, express costimulatory molecules, and produce inflammatory cytokines was assessed.
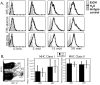
Results: Normal naïve T cells primed by EtOH-exposed DCs showed decreased proliferation in vitro and in vivo, compared to water-fed control mice. These EtOH-exposed DCs, after activation by CpG or tumor necrosis factor alpha (TNFα), were less able to upregulate costimulatory molecules CD40, CD80, or CD86, and produced less IL-12 p40, TNFα, and IFNα than DCs from water-fed mice. TLR9 and TNF receptor expression were also reduced in/on EtOH-exposed DCs. No evidence of defective Ag acquisition or processing as a result of EtOH feeding was identified.
Conclusions: Inadequate proliferation of normal T cells following stimulation by EtOH-exposed DCs is likely a result of diminished Signal 2 and Signal 3. Lack of adequate inflammatory stimulation of EtOH-exposed DCs because of diminished receptors for inflammatory mediators appears to be at least partially responsible for their dysfunction. These findings provide a mechanism to explain increased morbidity and mortality from infectious diseases in alcoholics and suggest targets for therapeutic intervention.
Copyright © 2010 by the Research Society on Alcoholism.
Figures





References
-
- Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–1914. - PubMed
-
- Aloman C, Gehring S, Wintermeyer P, Kuzushita N, Wands JR. Chronic ethanol consumption impairs cellular immune responses against HCV HS5 protein due to dendritic cell dysfunction. Gastroenterol. 2007;132:698–708. - PubMed
-
- Bafica A, Santiago HC, Goldszmid R, Ropert C, Gazzinelli RT, Sher A. TLR9 and TLR2 signaling together account for MyD88-dependent control of parasitemia in Trypanosoma cruzi infection. J Immunol. 2006;177:3515–3519. - PubMed
-
- Barchet W, Cella M, Colonna M. Plasmacytoid dendritic cells--virus experts of innate immunity. Semin Immunol. 2005;17:253–261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

