Population pharmacokinetic analysis of vancomycin in neonates. A new proposal of initial dosage guideline
- PMID: 21039765
- PMCID: PMC2997311
- DOI: 10.1111/j.1365-2125.2010.03736.x
Population pharmacokinetic analysis of vancomycin in neonates. A new proposal of initial dosage guideline
Abstract
Aim: To determine the population pharmacokinetic parameters of vancomycin in neonatal patients with a wide range of gestational age and birth weight, and subsequently to design an initial dosing schedule for vancomycin in neonates.
Methods: Using nonlinear mixed-effects modelling (NONMEM VI), the pharmacokinetics of vancomycin were investigated in 70 neonates with postmenstrual age and body weight ranging 25.1-48.1 weeks and 0.7-3.7kg, respectively. A one-compartment linear disposition model with zero order input and first-order elimination was used to describe the data. Nine demographic characteristics and 21 co-administered drugs were evaluated as covariates of clearance (CL) and distribution volume (V(d) ) of vancomycin.
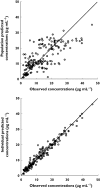
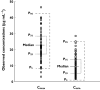
Results: Weight-normalized clearance of vancomycin was influenced by postmenstrual age (PMA) and co-administration of amoxicillin-clavulanic acid. Weight-normalized volume of distribution was influenced by co-administration of spironolactone. CL and V(d) of the typical individual in this study population (PMA = 34.6 weeks, weight = 1.7kg) were estimated to be 0.066lh(-1) kg(-1) (95% CI 0.059, 0.073lh(-1) kg(-1) ) and 0.572lkg(-1) (95% CI 0.505, 0.639lkg(-1) ), respectively. This model was used to predict a priori serum vancomycin concentrations in a validation group (n= 41), which were compared with observed concentrations to determine the predictive performance of the model. The 95% confidence interval of mean prediction error included zero for both peak and trough vancomycin concentrations.
Conclusions: Postmenstrual age, co-administration of amoxicillin-clavulanic acid and spironolactone have a significant effect on the weight-normalized CL and V(d) . An initial dosage guideline for vancomycin is proposed for preterm and full-term neonates, whereas the population pharmacokinetic model can be used for dosage individualization of vancomycin.
© 2010 The Authors. British Journal of Clinical Pharmacology © 2010 The British Pharmacological Society.
Figures

References
-
- Matzke GR. Vancomycin. In: Evans WE, Schentag JJ, Jusko WJ, editors. Applied Pharmacokinetics: Principles of Therapeutic Drug Monitoring. 3. Vancouver: Applied Therapeutics. Inc.; 1992. pp. 1–35. 15.
-
- Asbury WH, Darsey EH, Rose WB, Murphy JE, Buffington DE, Capers CC. Vancomycin pharmacokinetics in neonates and infants: a retrospective evaluation. Ann Pharmacother. 1993;27:490–6. - PubMed
-
- De Hoog M, Mouton JW, Van den Anker JN. Vancomycin. Pharmacokinetics and administration regimens in neonates. Clin Pharmacokinet. 2004;43:417–39. - PubMed
-
- De Hoog M, Schoemaker RC, Mouton JW, Van den Anker JN. Vancomycin population pharmacokinetics. Clin Pharmacol Ther. 2000;67:360–7. - PubMed
-
- Seay RE, Brundage RC, Jensen PD, Schilling CG, Edgren BE. Population pharmacokinetics of vancomycin in neonates. Clin Pharmacol Ther. 1994;56:169–75. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

