Neuropeptide-mediated calcium signaling in the suprachiasmatic nucleus network
- PMID: 21039959
- PMCID: PMC3059748
- DOI: 10.1111/j.1460-9568.2010.07411.x
Neuropeptide-mediated calcium signaling in the suprachiasmatic nucleus network
Abstract
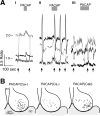
Neuroactive peptides and the intracellular calcium concentration ([Ca(2+) ](i) ) play important roles in light-induced modulation of gene expression in the suprachiasmatic nucleus (SCN) neurons that ultimately control behavioral rhythms. Vasoactive intestinal peptide (VIP) and arginine vasopressin (AVP) are expressed rhythmically within populations of SCN neurons. Pituitary adenylate cyclase-activating peptide (PACAP) is released from retinohypothalamic tract (RHT) terminals synapsing on SCN neurons. Nociceptin/orphanin FQ (OFQ) receptors are functionally expressed in the SCN. We examined the role of several neuropeptides on Ca(2+) signaling, simultaneously imaging multiple neurons within the SCN neural network. VIP reduced the [Ca(2+) ](i) in populations of SCN neurons during the day, but had little effect at night. Stimulation of the RHT at frequencies that simulate light input signaling evoked transient [Ca(2+) ](i) elevations that were not altered by VIP. AVP elevated the [Ca(2+) ](i) during both the day and night, PACAP produced variable responses, and OFQ induced a reduction in the [Ca(2+) ](i) similar to VIP. During the day, VIP lowered the [Ca(2+) ](i) to near nighttime levels, while AVP elevated [Ca(2+) ](i) during both the day and night, suggesting that the VIP effects on [Ca(2+) ](i) were dependent, and the AVP effects independent of the action potential firing activity state of the neuron. We hypothesize that VIP and AVP regulate, at least in part, Ca(2+) homeostasis in SCN neurons and may be a major point of regulation for SCN neuronal synchronization.
© 2010 The Authors. European Journal of Neuroscience © 2010 Federation of European Neuroscience Societies and Blackwell Publishing Ltd.
Figures



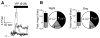


References
-
- Ajpru S, McArthur AJ, Piggins HD, Sugden D. Identification of PAC1 receptor isoform mRNAs by real-time PCR in rat suprachiasmatic nucleus. Brain Res Mol Brain Res. 2002;105:29–37. - PubMed
-
- Albers HE, Stopa EG, Zoeller RT, Kauer JS, King JC, Fink JS, Mobtaker H, Wolfe H. Day-night variation in prepro vasoactive intestinal peptide/peptide histidine isoleucine mRNA within the rat suprachiasmatic nucleus. Brain Res Mol Brain Res. 1990;7:85–89. - PubMed
-
- Albrecht U, Sun ZS, Eichele G, Lee CC. A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell. 1997;91:1055–1064. - PubMed
-
- Anton B, Fein J, To T, Li X, Silberstein L, Evans CJ. Immunohistochemical localization of ORL-1 in the central nervous system of the rat. The Journal of comparative neurology. 1996;368:229–251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

