Combining two potential causes of metalloproteinase secretion causes abdominal aortic aneurysms in rats: a new experimental model
- PMID: 21039990
- PMCID: PMC3052754
- DOI: 10.1111/j.1365-2613.2010.00746.x
Combining two potential causes of metalloproteinase secretion causes abdominal aortic aneurysms in rats: a new experimental model
Abstract
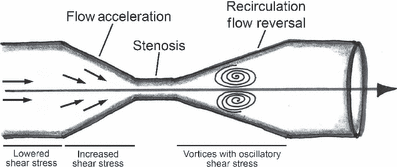
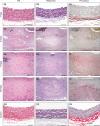
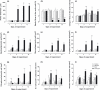
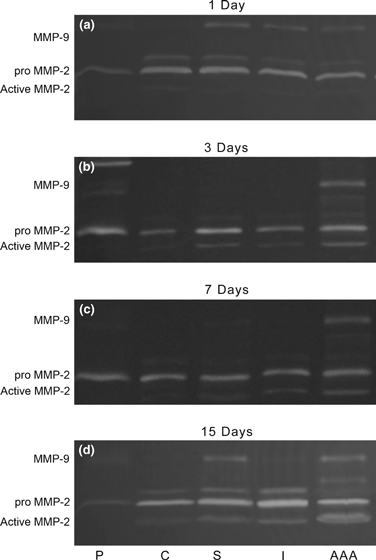
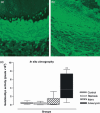
Progress in understanding the pathophysiology of abdominal aortic aneurysms (AAA) is dependent in part on the development and application of effective animal models that recapitulate key aspects of the disease. The objective was to produce an experimental model of AAA in rats by combining two potential causes of metalloproteinase (MMP) secretion: inflammation and turbulent blood flow. Male Wistar rats were randomly divided in four groups: Injury, Stenosis, Aneurysm and Control (40/group). The Injury group received a traumatic injury to the external aortic wall. The Stenosis group received an extrinsic stenosis at a corresponding location. The Aneurysm group received both the injury and stenosis simultaneously, and the Control group received a sham operation. Animals were euthanized at days 1, 3, 7 and 15. Aorta and/or aneurysms were collected and the fragments were fixed for morphologic, immunohistochemistry and morphometric analyses or frozen for MMP assays. AAAs had developed by day 3 in 60-70% of the animals, reaching an aortic dilatation ratio of more than 300%, exhibiting intense wall remodelling initiated at the adventitia and characterized by an obvious inflammatory infiltrate, mesenchymal proliferation, neoangiogenesis, elastin degradation and collagen deposition. Immunohistochemistry and zymography studies displayed significantly increased expressions of MMP-2 and MMP-9 in aneurysm walls compared to other groups. The haemo-dynamic alterations caused by the stenosis may have provided additional contribution to the MMPs liberation. This new model illustrated that AAA can be multifactorial and confirmed the key roles of MMP-2 and MMP-9 in this dynamic remodelling process.
© 2010 The Authors. International Journal of Experimental Pathology © 2010 International Journal of Experimental Pathology.
Figures



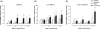
References
-
- Anidjar S, Salzmann JL, Gentric D, Lagneau P, Camilleri JP, Michel JB. Elastase-induced experimental aneurysms in rats. Circulation. 1990;82:973–981. - PubMed
-
- Aoki H, Yoshimura K, Matsuzaki M. Turning back the clock: regression of abdominal aortic aneurysms via pharmacotherapy. J. Mol. Med. 2007;85:1077–1088. - PubMed
-
- Bigatel DA, Elmorc JR, Carey DJ, Cizlncci SG, Franklin DR, Youkcy JR. The matrix metalloproteinase inhibitor BB-94 lintits expansion of experimental abdontinal aortic ancuwsms. J. Vasc. Surg. 1999;29:130–138. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

