Murine rVEGF164b, an inhibitory VEGF reduces VEGF-A-dependent endothelial proliferation and barrier dysfunction
- PMID: 21040119
- PMCID: PMC3057765
- DOI: 10.1111/j.1549-8719.2010.00047.x
Murine rVEGF164b, an inhibitory VEGF reduces VEGF-A-dependent endothelial proliferation and barrier dysfunction
Erratum in
- Microcirculation. 2010 Nov;17(8):669
Abstract
Objective: To investigate the effects of the murine inhibitory vascular endothelial growth factor (VEGF, rVEGF164b), we generated an adenoviral vector encoding rVEGF164b, and examined its effects on endothelial barrier, growth, and structure.
Method: Mouse vascular endothelial cells (MVEC) proliferation was determined by an MTT assay. Barrier of MVEC monolayers was measured by trans-endothelial electrical resistance (TEER). Reorganization of actin and zonula occludens-1 (ZO-1) were determined by fluorescent microscopy.
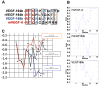
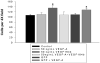
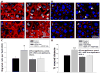
Results: Mouse venous endothelial cells treated with murine VEGF-A (VEGF-A) (50 ng/mL) increased proliferation (60.7 ± 0.1%) within 24 hours (p < 0.05) and rVEGF164b inhibited VEGF-A-induced proliferation. TEER was significantly decreased by VEGF-A (81.7 ± 6.2% of control). Treatment with rVEGF164b at 50 ng/mL transiently reduced MVEC barrier (p < 0.05) at 30 minutes post-treatment (87.9 ± 1.7% of control TEER), and returned to control levels by 40 minutes post-treatment. Treatment with rVEGF164b prevented barrier changes by subsequent exposure to VEGF-A. Treatment of MVECS with VEGF-A reorganized F-actin and ZO-1, which was attenuated by rVEGF164b.
Conclusions: VEGF-A may dysregulate endothelial barrier through junctional cytoskeleton processes, which can be attenuated by rVEGF164b. The VEGF-A stimulated MVEC proliferation, barrier dysregulation, and cytoskeletal rearrangement. However, rVEGF164b blocks these effects, therefore it may be useful for regulation studies of VEGF-A/VEGF-R signaling in many different models.
© 2010 John Wiley & Sons Ltd.
Figures



References
-
- Woolard J, Wang WY, Bevan HS, Qiu Y, Morbidelli L, Pritchard-Jones RO, Cui TG, Sugiono M, Waine E, Perrin R, et al. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res. 2004;64(21):7822–35. - PubMed
-
- Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7(5):359–71. - PubMed
-
- Appleton I, Brown NJ, Willis D, Colville-Nash PR, Alam C, Brown JR, Willoughby DA. The role of vascular endothelial growth factor in a murine chronic granulomatous tissue air pouch model of angiogenesis. J Pathol. 1996;180(1):90–4. - PubMed
-
- Ten Brick Robin A, C DT, Martin Jeremy L, Moline Karl V, Bergman Jeffery W, Cupp Andrea S. Vascular Endothelial Growth Factor Inhibitory Isoform Is Regulated Prior to Ovulation. Nebraska Beef Cattle Reports. 2007;1(1):113–115.
-
- Usui T, Ishida S, Yamashiro K, Kaji Y, Poulaki V, Moore J, Moore T, Amano S, Horikawa Y, Dartt D, et al. VEGF164(165) as the pathological isoform: differential leukocyte and endothelial responses through VEGFR1 and VEGFR2. Invest Ophthalmol Vis Sci. 2004;45(2):368–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

