Phylogeny informs ontogeny: a proposed common theme in the arterial pole of the vertebrate heart
- PMID: 21040422
- PMCID: PMC4180519
- DOI: 10.1111/j.1525-142X.2010.00441.x
Phylogeny informs ontogeny: a proposed common theme in the arterial pole of the vertebrate heart
Abstract
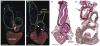
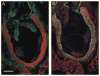
In chick and mouse embryogenesis, a population of cells described as the secondary heart field (SHF) adds both myocardium and smooth muscle to the developing cardiac outflow tract (OFT). Following this addition, at approximately HH stage 22 in chick embryos, for example, the SHF can be identified architecturally by an overlapping seam at the arterial pole, where beating myocardium forms a junction with the smooth muscle of the arterial system. Previously, using either immunohistochemistry or nitric oxide indicators such as diaminofluorescein 2-diacetate, we have shown that a similar overlapping architecture also exists in the arterial pole of zebrafish and some shark species. However, although recent work suggests that development of the zebrafish OFT may also proceed by addition of a SHF-like population of cells, the presence of a true SHF in zebrafish and in many other developmental biological models remains an open question. We performed a comprehensive morphological study of the OFT of a wide range of vertebrates. Our data suggest that all vertebrates possess three fundamental OFT components: a proximal myocardial component, a distal smooth muscle component, and a middle component that contains overlapping myocardium and smooth muscle surrounding and supporting the outflow valves. Because the middle OFT component of avians and mammals is derived from the SHF, our observations suggest that a SHF may be an evolutionarily conserved theme in vertebrate embryogenesis.
© 2010 Wiley Periodicals, Inc.
Figures

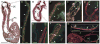






References
-
- Abu-Issa R, Kirby ML. Heart field: from Mesoderm to heart tube. Annu Rev Cell Dev Biol. 2007;23:45–68. - PubMed
-
- Anderson RH, Wilkinson JL, Becker AE. The bulbus cordis—a misunderstood region of the developing human heart: its significance to the classification of congenital cardiac malformations. Birth Defects Orig Artic Ser. 1978;14:1–28. - PubMed
-
- Bartelings MM, Gittenberger-de Groot AC. The outflow tract of the heart - embryologic and morphologic correlations. Int J Cardiol. 1989;22:289–300. - PubMed
-
- Blitz IL, Andelfinger G, Horb ME. Germ layers to organs: using Xenopus to study “later” development. Semin Cell Dev Biol. 2006;17:133–145. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

