Telocytes in human isolated atrial amyloidosis: ultrastructural remodelling
- PMID: 21040457
- PMCID: PMC3822724
- DOI: 10.1111/j.1582-4934.2010.01200.x
Telocytes in human isolated atrial amyloidosis: ultrastructural remodelling
Abstract
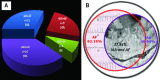
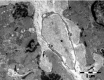
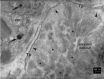
The human heart can be frequently affected by an organ-limited amyloidosis called isolated atrial amyloidosis (IAA). IAA is a frequent histopathological finding in patients with long-standing atrial fibrillation (AF). The aim of this paper was to investigate the ultrastructure of cardiomyocytes and telocytes in patients with AF and IAA. Human atrial biopsies were obtained from 37 patients undergoing cardiac surgery, 23 having AF (62%). Small fragments were harvested from the left and right atrial appendages and from the atrial sleeves of pulmonary veins and processed for electron microscopy (EM). Additional fragments were paraffin embedded for Congo-red staining. The EM examination certified that 17 patients had IAA and 82% of them had AF. EM showed that amyloid deposits, composed of characteristic 10-nm-thick filaments were strictly extra-cellular. Although, under light microscope some amyloid deposits seemed to be located within the cardiomyocyte cytoplasm, EM showed that these deposits are actually located in interstitial recesses. Moreover, EM revealed that telopodes, the long and slender processes of telocytes, usually surround the amyloid deposits limiting their spreading into the interstitium. Our results come to endorse the presumptive association of AF and IAA, and show the exclusive, extracellular localization of amyloid fibrils. The particular connection of telopodes with amyloid deposits suggests their involvement in isolated atrial amyloidosis and AF pathogenesis.
© 2010 The Authors Journal compilation © 2010 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures









Similar articles
-
Morpho-functional changes of cardiac telocytes in isolated atrial amyloidosis in patients with atrial fibrillation.Sci Rep. 2021 Feb 11;11(1):3563. doi: 10.1038/s41598-021-82554-0. Sci Rep. 2021. PMID: 33574429 Free PMC article.
-
Amyloid deposition as a cause of atrial remodelling in persistent valvular atrial fibrillation.Eur Heart J. 2004 Jul;25(14):1237-41. doi: 10.1016/j.ehj.2004.04.007. Eur Heart J. 2004. PMID: 15246642
-
Atrial amyloidosis: an arrhythmogenic substrate for persistent atrial fibrillation.Circulation. 2002 Oct 15;106(16):2091-7. doi: 10.1161/01.cir.0000034511.06350.df. Circulation. 2002. PMID: 12379579
-
Histological substrate of human atrial fibrillation.Biomed Pharmacother. 2010 Mar;64(3):177-83. doi: 10.1016/j.biopha.2009.09.017. Epub 2009 Nov 18. Biomed Pharmacother. 2010. PMID: 20006465 Review.
-
Human atrial fibrillation substrate: towards a specific fibrotic atrial cardiomyopathy.Eur Heart J. 2013 Sep;34(35):2731-8. doi: 10.1093/eurheartj/eht194. Epub 2013 Jun 11. Eur Heart J. 2013. PMID: 23761394 Review.
Cited by
-
Telocytes in regenerative medicine.J Cell Mol Med. 2015 Jul;19(7):1441-54. doi: 10.1111/jcmm.12594. Epub 2015 Jun 8. J Cell Mol Med. 2015. PMID: 26059693 Free PMC article. Review.
-
Cardiac telocytes are double positive for CD34/PDGFR-α.J Cell Mol Med. 2015 Aug;19(8):2036-42. doi: 10.1111/jcmm.12615. Epub 2015 Jun 17. J Cell Mol Med. 2015. PMID: 26082061 Free PMC article.
-
Transmission electron microscope evidence of telocytes in canine dura mater.J Cell Mol Med. 2016 Jan;20(1):188-92. doi: 10.1111/jcmm.12726. J Cell Mol Med. 2016. PMID: 26781033 Free PMC article.
-
Telocyte dynamics in psoriasis.J Cell Mol Med. 2015 Jul;19(7):1504-19. doi: 10.1111/jcmm.12601. Epub 2015 May 19. J Cell Mol Med. 2015. PMID: 25991475 Free PMC article.
-
Telocytes/CD34+ Stromal Cells in Pathologically Affected White Adipose Tissue.Int J Mol Sci. 2020 Dec 18;21(24):9694. doi: 10.3390/ijms21249694. Int J Mol Sci. 2020. PMID: 33353193 Free PMC article. Review.
References
-
- Westermark P, Benson MD, Buxbaum JN, et al. A primer of amyloid nomenclature. Amyloid. 2007;14:179–83. - PubMed
-
- Falk RH, Dubrey SW. Amyloid heart disease. Prog Cardiovasc Dis. 2010;52:347–61. - PubMed
-
- Collins AB, Smith RN, Stone JR. Classification of amyloid deposits in diagnostic cardiac specimens by immunofluorescence. Cardiovasc Pathol. 2008;18:205–6. - PubMed
-
- Rocken C, Peters B, Juenemann G, et al. Atrial amyloidosis. An arrhytmogenic substrate for persistent atrial fibrillation. Circulation. 2002;106:2091–7. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

