Putative γ-aminobutyric acid neurons in the ventral tegmental area have a similar pattern of plasticity as dopamine neurons during appetitive and aversive learning
- PMID: 21040517
- PMCID: PMC3070471
- DOI: 10.1111/j.1460-9568.2010.07371.x
Putative γ-aminobutyric acid neurons in the ventral tegmental area have a similar pattern of plasticity as dopamine neurons during appetitive and aversive learning
Abstract
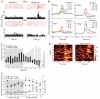
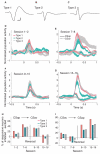
Dopamine influences affective, motor and cognitive processing, and multiple forms of learning and memory. This multifaceted functionality, which operates across long temporal windows, is broader than the narrow and temporally constrained role often ascribed to dopamine neurons as reward prediction error detectors. Given the modulatory nature of dopamine neurotransmission, that dopamine release is activated by both aversive and appetitive stimuli, and that dopamine receptors are often localized extrasynaptically, a role for dopamine in transmitting precise error signals has been questioned. Here we recorded from ventral tegmental area (VTA) neurons, while exposing rats to novel stimuli that were predictive of an appetitive or aversive outcome in the same behavioral session. The VTA contains dopamine and -aminobutyric acid (GABA) neurons that project to striatal and cortical regions and are strongly implicated in learning and affective processing. The response of VTA neurons, regardless of whether they had putative dopamine or GABA waveforms, transformed flexibly as animals learned to associate novel stimuli from different sensory modalities to appetitive or aversive outcomes. Learning the appetitive association led to larger excitatory VTA responses, whereas acquiring the aversive association led to a biphasic response of brief excitation followed by sustained inhibition. These responses shifted rapidly as outcome contingencies changed. These data suggest that VTA neurons interface sensory information with representational memory of aversive and appetitive events. This pattern of plasticity was not selective for putative dopamine neurons and generalized to other cells, suggesting that the temporally precise information transfer from the VTA may be mediated by faster acting GABA neurons.
Figures


Similar articles
-
Coordinated activity of ventral tegmental neurons adapts to appetitive and aversive learning.PLoS One. 2012;7(1):e29766. doi: 10.1371/journal.pone.0029766. Epub 2012 Jan 6. PLoS One. 2012. PMID: 22238652 Free PMC article.
-
The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses.Neuron. 2009 Mar 12;61(5):786-800. doi: 10.1016/j.neuron.2009.02.001. Neuron. 2009. PMID: 19285474 Free PMC article.
-
Glutamatergic Ventral Pallidal Neurons Modulate Activity of the Habenula-Tegmental Circuitry and Constrain Reward Seeking.Biol Psychiatry. 2018 Jun 15;83(12):1012-1023. doi: 10.1016/j.biopsych.2018.01.003. Epub 2018 Jan 12. Biol Psychiatry. 2018. PMID: 29452828 Free PMC article.
-
Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement.Annu Rev Neurosci. 2007;30:289-316. doi: 10.1146/annurev.neuro.30.051606.094341. Annu Rev Neurosci. 2007. PMID: 17376009 Review.
-
Involvement of basal ganglia and orbitofrontal cortex in goal-directed behavior.Prog Brain Res. 2000;126:193-215. doi: 10.1016/S0079-6123(00)26015-9. Prog Brain Res. 2000. PMID: 11105648 Review.
Cited by
-
Financial gain- and loss-related BOLD signals in the human ventral tegmental area and substantia nigra pars compacta.Eur J Neurosci. 2019 May;49(9):1196-1209. doi: 10.1111/ejn.14288. Epub 2018 Dec 13. Eur J Neurosci. 2019. PMID: 30471149 Free PMC article.
-
Distinct prestimulus and poststimulus activation of VTA neurons correlates with stimulus detection.J Neurophysiol. 2013 Jul;110(1):75-85. doi: 10.1152/jn.00784.2012. Epub 2013 Apr 3. J Neurophysiol. 2013. PMID: 23554430 Free PMC article.
-
Mesoaccumbal Dopamine Heterogeneity: What Do Dopamine Firing and Release Have to Do with It?Annu Rev Neurosci. 2022 Jul 8;45:109-129. doi: 10.1146/annurev-neuro-110920-011929. Epub 2022 Feb 28. Annu Rev Neurosci. 2022. PMID: 35226827 Free PMC article. Review.
-
Disrupted Neuronal Dynamics of Reward Encoding in the Medial Prefrontal Cortex and the Ventral Tegmental Area after Episodic Social Stress.eNeuro. 2025 Jul 25;12(7):ENEURO.0229-25.2025. doi: 10.1523/ENEURO.0229-25.2025. Print 2025 Jul. eNeuro. 2025. PMID: 40664513 Free PMC article.
-
Optogenetic evidence that pallidal projections, not nigral projections, from the nucleus accumbens core are necessary for reinstating cocaine seeking.J Neurosci. 2013 Aug 21;33(34):13654-62. doi: 10.1523/JNEUROSCI.1570-13.2013. J Neurosci. 2013. PMID: 23966687 Free PMC article.
References
-
- Anstrom KK, Woodward DJ. Restraint increases dopaminergic burst firing in awake rats. Neuropsychopharmacology. 2005;30:1832–1840. - PubMed
-
- Beninger RJ. The role of dopamine in locomotor activity and learning. Brain Res. Rev. 1983;6:173–196. - PubMed
-
- Carr DB, Sesack SR. GABA-containing neurons in the rat ventral tegmental area project to the prefrontal cortex. Synapse. 2000;38:114–123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

