Multidisciplinary and multifaceted outpatient management of patients with osteoarthritis: protocol for a randomised, controlled trial
- PMID: 21040556
- PMCID: PMC2989945
- DOI: 10.1186/1471-2474-11-253
Multidisciplinary and multifaceted outpatient management of patients with osteoarthritis: protocol for a randomised, controlled trial
Abstract
Background: Osteoarthritis (OA) is a prevalent joint disorder with a need for efficient and evidence-based management strategies.
Objectives: The primary purpose of this study is to compare the effects of a multidisciplinary outpatient clinic, including a brief group-based educational programme, with a traditional individual outpatient clinic for patients with hip, knee, hand or generalized OA. A secondary purpose is to investigate the effects of a telephone follow-up call.
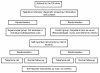
Methods: This is a pragmatic randomised single-blind controlled study with a total of 400 patients with hip, knee, hand or generalized OA between 40 and 80 years referred to an outpatient rheumatology hospital clinic. The randomisation is stratified according to the diagnostic subgroups. The experimental group is exposed to a multidisciplinary and multifaceted intervention, including a 3.5 hour group-based patient education programme about OA in addition to individual consultations with members of a multidisciplinary team. The control intervention is based on regular care with an individual outpatient consultation with a rheumatologist (treatment as usual). Primary outcomes are patient satisfaction measured at 4 months and cost-effectiveness measured at 12 months. Secondary outcomes are pain and global disease activity measured on a numeric rating scales (NRS), generic and disease specific functioning and disability using Short Form-36 (SF-36) health survey, the Western Ontario and McMaster Universities Osteoarthritis Index 3 (WOMAC), the Australian/Canadian Osteoarthritis Hand Index (AUSCAN), and a patient-generated measure of disability (Patient-Specific Functional scale, PSFS). Global perceived effect of change in health status during the study period is also reported. At 4-month follow-up, patients in both groups will be randomly allocated to a 10-minute telephone call or no follow-up ("treatment as usual"). After additional 8 months (12-month follow-up) the four groups will be compared in a secondary analysis with regard to health outcomes and health care costs.
Discussion: This trial will provide results on how multidisciplinary and multifaceted management of patients with OA affects health outcomes and health care costs.
Trial registration: Current Controlled Trials ISRCTN25778426.
Figures
References
-
- Jordan KM, Arden NK, Doherty M, Bannwarth B, Bijlsma JW, Dieppe P, Gunther K, Hauselmann H, Herrero-Beaumont G, Kaklamanis P, Lohmander S, Leeb B, Lequesne M, Mazieres B, Martin-Mola E, Pavelka K, Pendleton A, Punzi L, Serni U, Swoboda B, Verbruggen G, Zimmerman-Gorska I, Dougados M. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT) Ann Rheum Dis. 2003;62(12):1145–55. doi: 10.1136/ard.2003.011742. - DOI - PMC - PubMed
-
- Zhang W, Doherty M, Arden N, Bannwarth B, Bijlsma J, Gunther KP, Hauselmann HJ, Herrero-Beaumont G, Jordan K, Kaklamanis P, Leeb B, Lequesne M, Lohmander S, Mazieres B, Martin-Mola E, Pavelka K, Pendleton A, Punzi L, Swoboda B, Varatojo R, Verbruggen G, Zimmermann-Gorska I, Dougados M. EULAR evidence based recommendations for the management of hip osteoarthritis: report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT) Ann Rheum Dis. 2005;64(5):669–81. doi: 10.1136/ard.2004.028886. - DOI - PMC - PubMed
-
- Hawley DJ, Wolfe F. Pain, disability, and pain/disability relationships in seven rheumatic disorders: a study of 1,522 patients. J Rheumatol. 1991;18(10):1552–7. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous