Designing and engineering evolutionary robust genetic circuits
- PMID: 21040586
- PMCID: PMC2991278
- DOI: 10.1186/1754-1611-4-12
Designing and engineering evolutionary robust genetic circuits
Abstract
Background: One problem with engineered genetic circuits in synthetic microbes is their stability over evolutionary time in the absence of selective pressure. Since design of a selective environment for maintaining function of a circuit will be unique to every circuit, general design principles are needed for engineering evolutionary robust circuits that permit the long-term study or applied use of synthetic circuits.
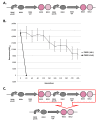
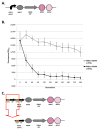
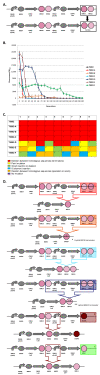
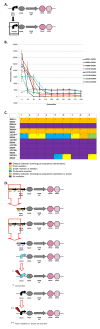
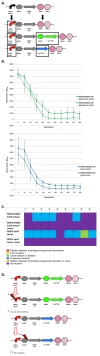
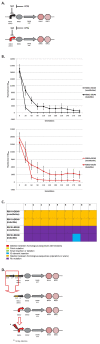
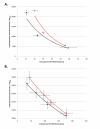
Results: We first measured the stability of two BioBrick-assembled genetic circuits propagated in Escherichia coli over multiple generations and the mutations that caused their loss-of-function. The first circuit, T9002, loses function in less than 20 generations and the mutation that repeatedly causes its loss-of-function is a deletion between two homologous transcriptional terminators. To measure the effect between transcriptional terminator homology levels and evolutionary stability, we re-engineered six versions of T9002 with a different transcriptional terminator at the end of the circuit. When there is no homology between terminators, the evolutionary half-life of this circuit is significantly improved over 2-fold and is independent of the expression level. Removing homology between terminators and decreasing expression level 4-fold increases the evolutionary half-life over 17-fold. The second circuit, I7101, loses function in less than 50 generations due to a deletion between repeated operator sequences in the promoter. This circuit was re-engineered with different promoters from a promoter library and using a kanamycin resistance gene (kanR) within the circuit to put a selective pressure on the promoter. The evolutionary stability dynamics and loss-of-function mutations in all these circuits are described. We also found that on average, evolutionary half-life exponentially decreases with increasing expression levels.
Conclusions: A wide variety of loss-of-function mutations are observed in BioBrick-assembled genetic circuits including point mutations, small insertions and deletions, large deletions, and insertion sequence (IS) element insertions that often occur in the scar sequence between parts. Promoter mutations are selected for more than any other biological part. Genetic circuits can be re-engineered to be more evolutionary robust with a few simple design principles: high expression of genetic circuits comes with the cost of low evolutionary stability, avoid repeated sequences, and the use of inducible promoters increases stability. Inclusion of an antibiotic resistance gene within the circuit does not ensure evolutionary stability.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

