Novel insights into molecular mechanisms of abruption-induced preterm birth
- PMID: 21040617
- PMCID: PMC4792258
- DOI: 10.1017/S1462399410001675
Novel insights into molecular mechanisms of abruption-induced preterm birth
Abstract
Preterm birth (PTB) complicates more than 12% of all deliveries. Despite significant research, the aetiology of most cases of PTB remains elusive. Two major antecedents of PTB, intra-amniotic infection and decidual haemorrhage (abruption), can exhibit dissimilar demographic and genetic predispositions, despite sharing common molecular and cellular pathways. The use of high-throughput, high-dimensional technologies reveals substantial crosstalk between the coagulation and inflammation pathways. Tissue factor, thrombin and cytokines are key mediators of this crosstalk. Abruptions are associated with excess thrombin generated from decidual-cell-expressed tissue factor. Although thrombin is a primary mediator of the coagulation cascade, it can also promote inflammation-associated PTB by enhancing expression of matrix metalloproteinase and neutrophil-chemoattracting and -activating chemokines. Here, we provide novel insights into the molecular mechanisms and pathways leading to PTB in the setting of placental abruption.
Figures

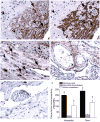

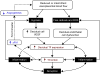
References
-
- Stoll BJ, et al. National Institute of Child Health and Human Development Neonatal Research Network. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. Journal of the American Medical Association. 2004;292:2357–2365. - PubMed
-
- Zerhouni EA. US biomedical research: basic, translational, and clinical sciences. Journal of the American Medical Association. 2005;294:1352–1358. - PubMed
-
- McLean M, et al. A placental clock controlling the length of human pregnancy. Nature Medicine. 1995;1:460–463. - PubMed
-
- Zenclussen AC, et al. Immunology of pregnancy: cellular mechanisms allowing fetal survival within the maternal uterus. Expert Reviews in Molecular Medicine. 2007;9:1–14. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

