Small G protein signaling in neuronal plasticity and memory formation: the specific role of ras family proteins
- PMID: 21040840
- PMCID: PMC3008420
- DOI: 10.1016/j.neuron.2010.09.013
Small G protein signaling in neuronal plasticity and memory formation: the specific role of ras family proteins
Abstract
Small G proteins are an extensive family of proteins that bind and hydrolyze GTP. They are ubiquitous inside cells, regulating a wide range of cellular processes. Recently, many studies have examined the role of small G proteins, particularly the Ras family of G proteins, in memory formation. Once thought to be primarily involved in the transduction of a variety of extracellular signals during development, it is now clear that Ras family proteins also play critical roles in molecular processing underlying neuronal and behavioral plasticity. We here review a number of recent studies that explore how the signaling of Ras family proteins contributes to memory formation. Understanding these signaling processes is of fundamental importance both from a basic scientific perspective, with the goal of providing mechanistic insights into a critical aspect of cognitive behavior, and from a clinical perspective, with the goal of providing effective therapies for a range of disorders involving cognitive impairments.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
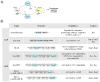
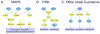


References
-
- Adams JP, Sweatt JD. Molecular psychology: roles for the ERK MAP kinase cascade in memory. Annu Rev Pharmacol Toxicol. 2002;42:135–163. - PubMed
-
- Ahn YH, Han JH, Hong SH. Rap1 and p38 MAPK mediate 8-chloro-cAMP-induced growth inhibition in mouse fibroblast DT cells. Journal of cellular physiology. 2006;209:1039–1045. - PubMed
-
- Alpar A, Gartner U, Seeger G, Hartig W, Brauer K, Arendt T. Constitutive expression of p21H-ras(Val12) in pyramidal neurons results in reorganization of mouse neocortical afferents. Journal of neurobiology. 2004;60:263–274. - PubMed
-
- Alpar A, Palm K, Schierwagen A, Arendt T, Gartner U. Expression of constitutively active p21H-rasval12 in postmitotic pyramidal neurons results in increased dendritic size and complexity. The Journal of comparative neurology. 2003;467:119–133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

