Cortical preparatory activity: representation of movement or first cog in a dynamical machine?
- PMID: 21040842
- PMCID: PMC2991102
- DOI: 10.1016/j.neuron.2010.09.015
Cortical preparatory activity: representation of movement or first cog in a dynamical machine?
Abstract
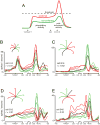
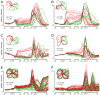
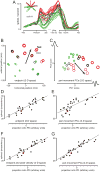
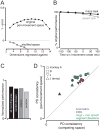
The motor cortices are active during both movement and movement preparation. A common assumption is that preparatory activity constitutes a subthreshold form of movement activity: a neuron active during rightward movements becomes modestly active during preparation of a rightward movement. We asked whether this pattern of activity is, in fact, observed. We found that it was not: at the level of a single neuron, preparatory tuning was weakly correlated with movement-period tuning. Yet, somewhat paradoxically, preparatory tuning could be captured by a preferred direction in an abstract "space" that described the population-level pattern of movement activity. In fact, this relationship accounted for preparatory responses better than did traditional tuning models. These results are expected if preparatory activity provides the initial state of a dynamical system whose evolution produces movement activity. Our results thus suggest that preparatory activity may not represent specific factors, and may instead play a more mechanistic role.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures



References
-
- Bastian A, Riehle A, Erlhagen W, Schoner G. Prior information preshapes the population representation of movement direction in motor cortex. Neuroreport. 1998;9:315–319. - PubMed
-
- Bastian A, Schoner G, Riehle A. Preshaping and continuous evolution of motor cortical representations during movement preparation. Eur J Neurosci. 2003;18:2047–2058. - PubMed
-
- Batista AP, Santhanam G, Yu BM, Ryu SI, Afshar A, Shenoy KV. Reference frames for reach planning in macaque dorsal premotor cortex. J Neurophysiol. 2007;98:966–983. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

