Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging
- PMID: 21040844
- PMCID: PMC3056408
- DOI: 10.1016/j.neuron.2010.09.043
Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging
Erratum in
-
Pericytes Control Key Neurovascular Functions and Neuronal Phenotype in the Adult Brain and during Brain Aging.Neuron. 2023 Oct 4;111(19):3131-3132. doi: 10.1016/j.neuron.2023.09.007. Neuron. 2023. PMID: 37797582 Free PMC article. No abstract available.
Abstract
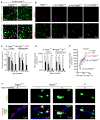
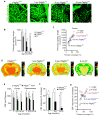
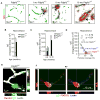
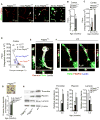
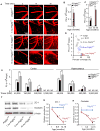
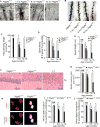
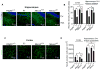
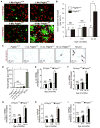
Pericytes play a key role in the development of cerebral microcirculation. The exact role of pericytes in the neurovascular unit in the adult brain and during brain aging remains, however, elusive. Using adult viable pericyte-deficient mice, we show that pericyte loss leads to brain vascular damage by two parallel pathways: (1) reduction in brain microcirculation causing diminished brain capillary perfusion, cerebral blood flow, and cerebral blood flow responses to brain activation that ultimately mediates chronic perfusion stress and hypoxia, and (2) blood-brain barrier breakdown associated with brain accumulation of serum proteins and several vasculotoxic and/or neurotoxic macromolecules ultimately leading to secondary neuronal degenerative changes. We show that age-dependent vascular damage in pericyte-deficient mice precedes neuronal degenerative changes, learning and memory impairment, and the neuroinflammatory response. Thus, pericytes control key neurovascular functions that are necessary for proper neuronal structure and function, and pericyte loss results in a progressive age-dependent vascular-mediated neurodegeneration.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures

Comment in
-
Pericytes: blood-brain barrier safeguards against neurodegeneration?Neuron. 2010 Nov 4;68(3):321-3. doi: 10.1016/j.neuron.2010.10.024. Neuron. 2010. PMID: 21040834
References
-
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. - PubMed
-
- Bauer HC, Bauer H, Lametschwandtner A, Amberger A, Ruiz P, Steiner M. Neovascularization and the appearance of morphological characteristics of the blood-brain barrier in the embryonic mouse central nervous system. Brain Res Dev Brain Res. 1993;75:269–278. - PubMed
-
- Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

