The steroid hormone ecdysone functions with intrinsic chromatin remodeling factors to control female germline stem cells in Drosophila
- PMID: 21040900
- PMCID: PMC3292427
- DOI: 10.1016/j.stem.2010.10.001
The steroid hormone ecdysone functions with intrinsic chromatin remodeling factors to control female germline stem cells in Drosophila
Abstract
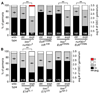
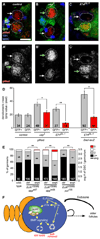
Steroid hormones are known systemic regulators of multiple normal and cancerous tissues; however, whether or how they impact the fate and function of adult stem cells is unclear. In the Drosophila ovary, insulin signals modulate the proliferation and self-renewal of germline stem cells (GSCs), yet despite evidence that additional systemic factors control GSC activity, these have remained largely unknown. Here, we report that ecdysone, a steroid hormone structurally related to mammalian sex steroids, directly regulates adult GSC proliferation and self-renewal independently of insulin signaling. Ecdysone controls GSCs through a functional interaction with the chromatin remodeling factors ISWI, an intrinsic epigenetic factor required for GSC fate and activity, and Nurf301, the largest subunit of the ISWI-containing NURF chromatin remodeling complex. Our findings support a link between systemic steroid hormones and the intrinsic chromatin remodeling machinery as a potential mechanism to promote broad transcriptional programs required for adult stem cell self-renewal.
Copyright © 2010 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing conflicts of interest.
Figures
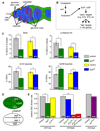
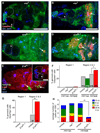
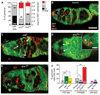
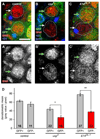
References
-
- Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, Yasuda H, Smyth GK, Martin TJ, Lindeman GJ, et al. Control of mammary stem cell function by steroid hormone signalling. Nature. 2010;465:798–802. - PubMed
-
- Bai J, Uehara Y, Montell DJ. Regulation of invasive cell behavior by taiman, a Drosophila protein related to AIB1, a steroid receptor coactivator amplified in breast cancer. Cell. 2000;103:1047–1058. - PubMed
-
- Bialecki M, Shilton A, Fichtenberg C, Segraves WA, Thummel CS. Loss of the ecdysteroid-inducible E75A orphan nuclear receptor uncouples molting from metamorphosis in Drosophila. Dev Cell. 2002;3:209–220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

