mTOR activation induces tumor suppressors that inhibit leukemogenesis and deplete hematopoietic stem cells after Pten deletion
- PMID: 21040901
- PMCID: PMC2995996
- DOI: 10.1016/j.stem.2010.09.015
mTOR activation induces tumor suppressors that inhibit leukemogenesis and deplete hematopoietic stem cells after Pten deletion
Abstract
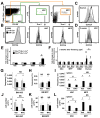
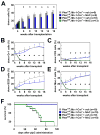
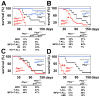
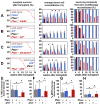
Pten deficiency depletes hematopoietic stem cells (HSCs) but expands leukemia-initiating cells, and the mTOR inhibitor, rapamycin, blocks these effects. Understanding the opposite effects of mTOR activation on HSCs versus leukemia-initiating cells could improve antileukemia therapies. We found that the depletion of Pten-deficient HSCs was not caused by oxidative stress and could not be blocked by N-acetyl-cysteine. Instead, Pten deletion induced, and rapamycin attenuated, the expression of p16(Ink4a) and p53 in HSCs, and p19(Arf) and p53 in other hematopoietic cells. p53 suppressed leukemogenesis and promoted HSC depletion after Pten deletion. p16(Ink4a) also promoted HSC depletion but had a limited role suppressing leukemogenesis. p19(Arf) strongly suppressed leukemogenesis but did not deplete HSCs. Secondary mutations attenuated this tumor suppressor response in some leukemias that arose after Pten deletion. mTOR activation therefore depletes HSCs by a tumor suppressor response that is attenuated by secondary mutations in leukemogenic clones.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures



References
-
- Aggerholm A, Gronbaek K, Guldberg P, Hokland P. Mutational analysis of the tumour suppressor gene MMAC1/PTEN in malignant myeloid disorders. European journal of haematology. 2000;65:109–113. - PubMed
-
- Akala OO, Park IK, Qian D, Pihalja M, Becker MW, Clarke MF. Long-term haematopoietic reconstitution by Trp53−/−p16Ink4a−/−p19Arf−/− multipotent progenitors. Nature. 2008;453:228–232. - PubMed
-
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. - PubMed
-
- Chang H, Qi XY, Claudio J, Zhuang L, Patterson B, Stewart AK. Analysis of PTEN deletions and mutations in multiple myeloma. Leukemia research. 2006;30:262–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

