Endothelial cells from humans and mice with polycystic kidney disease are characterized by polyploidy and chromosome segregation defects through survivin down-regulation
- PMID: 21041232
- PMCID: PMC3005905
- DOI: 10.1093/hmg/ddq470
Endothelial cells from humans and mice with polycystic kidney disease are characterized by polyploidy and chromosome segregation defects through survivin down-regulation
Abstract
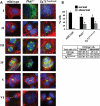
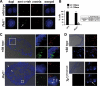
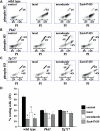
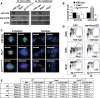
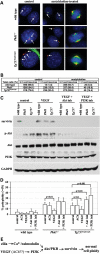
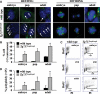
Autosomal-dominant polycystic kidney disease (ADPKD) is the most common hereditary and systemic disorder associated with various cardiovascular complications. It has been implicated with dysfunction in primary cilia. We and others have shown that the immediate function of endothelial cilia is to sense extracellular signal. The long-term function of cilia is hypothesized to regulate cell cycle. Here, we show that ciliary function (polycystins) and structure (polaris) are required for proper cellular division. Cilia mutant cells undergo abnormal cell division with apparent defects in mitotic spindle formation, cellular spindle assembly checkpoint and centrosome amplification. Down-regulation of the chromosomal passenger survivin contributes to these abnormalities, which further result in cell polyploidy. Re-expression of survivin restores a competent spindle assembly checkpoint and reduces polyploidy. Aged animals show a more severe phenotype in cellular division, consistent with progression of cardiovascular complications seen in older ADPKD patients. For the first time, we show that structure and function of mechanosensory cilia are crucial in maintaining proper cellular proliferation. Furthermore, developmental aging plays a crucial role in the progression of these abnormal cellular phenotypes. We propose that abnormal function or structure of primary cilia not only causes failure to transmit extracellular signals, but also is associated with cytokinesis defects in both mice and humans with polycystic kidney disease.
Figures




References
-
- Harris P.C., Torres V.E. Polycystic kidney disease. Annual Rev. Med. 2009;60:321–337. doi:10.1146/annurev.med.60.101707.125712. - DOI - PMC - PubMed
-
- AbouAlaiwi W.A., Takahashi M., Mell B.R., Jones T.J., Ratnam S., Kolb R.J., Nauli S.M. Ciliary polycystin-2 is a mechanosensitive calcium channel involved in nitric oxide signaling cascades. Circ. Res. 2009;104:860–869. doi:10.1161/CIRCRESAHA.108.192765. - DOI - PMC - PubMed
-
- Nauli S.M., Kawanabe Y., Kaminski J.J., Pearce W.J., Ingber D.E., Zhou J. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation. 2008;117:1161–1171. doi:10.1161/CIRCULATIONAHA.107.710111. - DOI - PMC - PubMed
-
- Poelmann R.E., Van der Heiden K., Gittenberger-de Groot A., Hierck B.P. Deciphering the endothelial shear stress sensor. Circulation. 2008;117:1124–1126. doi:10.1161/CIRCULATIONAHA.107.753889. - DOI - PubMed
-
- Nauli S.M., Alenghat F.J., Luo Y., Williams E., Vassilev P., Li X., Elia A.E., Lu W., Brown E.M., Quinn S.J., et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet. 2003;33:129–137. doi:10.1038/ng1076. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

