GRK5 deficiency accelerates {beta}-amyloid accumulation in Tg2576 mice via impaired cholinergic activity
- PMID: 21041302
- PMCID: PMC3009881
- DOI: 10.1074/jbc.M110.170894
GRK5 deficiency accelerates {beta}-amyloid accumulation in Tg2576 mice via impaired cholinergic activity
Abstract
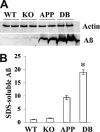
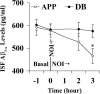
Membrane G protein-coupled receptor kinase 5 (GRK5) deficiency is linked to Alzheimer disease, yet its precise roles in the disease pathogenesis remain to be delineated. We have previously demonstrated that GRK5 deficiency selectively impairs desensitization of presynaptic M2 autoreceptors, which causes presynaptic M2 hyperactivity and inhibits acetylcholine release. Here we report that inactivation of one copy of Grk5 gene in transgenic mice overexpressing β-amyloid precursor protein (APP) carrying Swedish mutations (Tg2576 or APPsw) resulted in significantly increased β-amyloid (Aβ) accumulation, including increased Aβ(+) plaque burdens and soluble Aβ in brain lysates and interstitial fluid (ISF). In addition, secreted β-APP fragment (sAPPβ) also increased, whereas full-length APP level did not change, suggesting an alteration in favor of β-amyloidogenic APP processing in these animals. Reversely, perfusion of methoctramine, a selective M2 antagonist, fully corrected the difference between the control and GRK5-deficient APPsw mice for ISF Aβ. In contrast, a cholinesterase inhibitor, eserine, although significantly decreasing the ISF Aβ in both control and GRK5-deficient APPsw mice, failed to correct the difference between them. However, combining eserine with methoctramine additively reduced the ISF Aβ further in both animals. Altogether, these findings indicate that GRK5 deficiency accelerates β-amyloidogenic APP processing and Aβ accumulation in APPsw mice via impaired cholinergic activity and that presynaptic M2 hyperactivity is the specific target for eliminating the pathologic impact of GRK5 deficiency. Moreover, a combination of an M2 antagonist and a cholinesterase inhibitor may reach the maximal disease-modifying effect for both amyloid pathology and cholinergic dysfunction.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

