Identification and functional characterization of TMEM16A, a Ca2+-activated Cl- channel activated by extracellular nucleotides, in biliary epithelium
- PMID: 21041307
- PMCID: PMC3013035
- DOI: 10.1074/jbc.M110.164970
Identification and functional characterization of TMEM16A, a Ca2+-activated Cl- channel activated by extracellular nucleotides, in biliary epithelium
Abstract
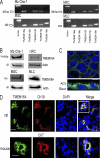
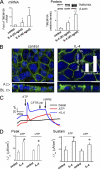
Cl(-) channels in the apical membrane of biliary epithelial cells (BECs) provide the driving force for ductular bile formation. Although a cystic fibrosis transmembrane conductance regulator has been identified in BECs and contributes to secretion via secretin binding basolateral receptors and increasing [cAMP](i), an alternate Cl(-) secretory pathway has been identified that is activated via nucleotides (ATP, UTP) binding apical P2 receptors and increasing [Ca(2+)](i). The molecular identity of this Ca(2+)-activated Cl(-) channel is unknown. The present studies in human, mouse, and rat BECs provide evidence that TMEM16A is the operative channel and contributes to Ca(2+)-activated Cl(-) secretion in response to extracellular nucleotides. Furthermore, Cl(-) currents measured from BECs isolated from distinct areas of intrahepatic bile ducts revealed important functional differences. Large BECs, but not small BECs, exhibit cAMP-stimulated Cl(-) currents. However, both large and small BECs express TMEM16A and exhibit Ca(2+)-activated Cl(-) efflux in response to extracellular nucleotides. Incubation of polarized BEC monolayers with IL-4 increased TMEM16A protein expression, membrane localization, and transepithelial secretion (I(sc)). These studies represent the first molecular identification of an alternate, noncystic fibrosis transmembrane conductance regulator, Cl(-) channel in BECs and suggest that TMEM16A may be a potential target to modulate bile formation in the treatment of cholestatic liver disorders.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

