Outcome selection and role of patient reported outcomes in contemporary cardiovascular trials: systematic review
- PMID: 21041324
- PMCID: PMC2967478
- DOI: 10.1136/bmj.c5707
Outcome selection and role of patient reported outcomes in contemporary cardiovascular trials: systematic review
Abstract
Objectives: To systematically assess the type of outcomes selected and the prevalence of patient reported outcomes in contemporary cardiovascular trials and to quantify any misuse or underuse of patient reported outcomes using a specially developed tool that would allow estimation of the relevance of such outcomes to clinical decision making.
Design: Systematic review.
Data sources: Medline and Embase.
Study selection: Randomised controlled trials of the treatment for or prevention of cardiovascular disease published in 10 leading general medical and cardiology journals from January 2005 to December 2008.
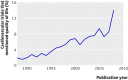
Results: Primary outcomes were patient important (death, morbidity, or patient reported outcomes) in only 93 of 413 trials (23%, SE 2%), whereas another 92 (22%, SE 2%) combined these outcomes with other less important ones into a composite. Sixty five trials (16%; SE 2%) used at least one instrument to measure patient reported outcomes, mostly in trials where such information would have been important or crucial for clinical decision making (52 trials). Patient reported outcomes were judged to be of little incremental value to a large number of, mostly explanatory, cardiovascular trials (152 trials). However, many trials in which patient reported outcomes would have been important or crucial for clinical decision making did not report such outcomes (122 of 174 trials, 70%). These included several trials that primarily aimed to improve symptoms or functional status, trials that tested interventions with a considerable potential for causing harm (mainly bleeding) that were not meaningfully measured, and trials with composite outcomes that were dominated by outcomes of questionable importance to patients.
Conclusions: Despite a continued rise in the reporting of patient reported outcomes with no evidence for their misuse in more recent cardiovascular trials, they seem to be still underused once their relevance to clinical decision making has been taken into account. This was largely explained by inappropriate use of composite outcomes and inadequate measurement of harms.
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form at
Figures
References
-
- Quality of life and clinical trials. Lancet 1995;346:1-2. - PubMed
-
- Acquadro C, Berzon R, Dubois D, Leidy NK, Marquis P, Revicki D, et al. Incorporating the patient’s perspective into drug development and communication: an ad hoc task force report of the Patient-Reported Outcomes (PRO) Harmonization Group meeting at the Food and Drug Administration, February 16, 2001. Value Health 2003;6:522-31. - PubMed
-
- McDermott MM, Liu K, Guralnik JM, Martin GJ, Criqui MH, Greenland P. Measurement of walking endurance and walking velocity with questionnaire: Validation of the walking impairment questionnaire in men and women with peripheral arterial disease. J Vasc Surg 1998;28:1072-81. - PubMed
-
- Guyatt GH, Nogradi S, Halcrow S, Singer J, Sullivan MJ, Fallen EL. Development and testing of a new measure of health status for clinical trials in heart failure. J Gen Intern Med 1989;4:101-7. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources