The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program
- PMID: 21041407
- PMCID: PMC2964748
- DOI: 10.1101/gad.1978810
The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program
Abstract
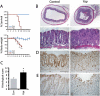
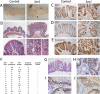
Although a developmental role for Hippo signaling in organ size control is well appreciated, how this pathway functions in tissue regeneration is largely unknown. Here we address this issue using a dextran sodium sulfate (DSS)-induced colonic regeneration model. We find that regenerating crypts express elevated Yes-associated protein (YAP) levels. Inactivation of YAP causes no obvious intestinal defects under normal homeostasis, but severely impairs DSS-induced intestinal regeneration. Conversely, hyperactivation of YAP results in widespread early-onset polyp formation following DSS treatment. Thus, the YAP oncoprotein must be exquisitely controlled in tissue regeneration to allow compensatory proliferation and prevent the intrinsic oncogenic potential of a tissue regeneration program.
Figures



Comment in
-
YAP tips the balance.Nat Rev Cancer. 2010 Dec;10(12):811. doi: 10.1038/nrc2973. Nat Rev Cancer. 2010. PMID: 21155175 No abstract available.
References
-
- Badouel C, Garg A, McNeill H 2009. Herding Hippos: Regulating growth in flies and man. Curr Opin Cell Biol 21: 837–843 - PubMed
-
- Beachy PA, Karhadkar SS, Berman DM 2004. Tissue repair and stem cell renewal in carcinogenesis. Nature 432: 324–331 - PubMed
-
- Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T, Nebelsiek T, Lundgren-May T, Canli O, Schwitalla S, et al. 2009. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell 15: 91–102 - PubMed
-
- Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, Brummelkamp TR 2007. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol 17: 2054–2060 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
