Delta40p53 controls the switch from pluripotency to differentiation by regulating IGF signaling in ESCs
- PMID: 21041409
- PMCID: PMC2964751
- DOI: 10.1101/gad.1987810
Delta40p53 controls the switch from pluripotency to differentiation by regulating IGF signaling in ESCs
Abstract
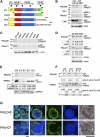
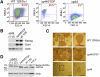
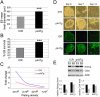
Δ40p53 is a transactivation-deficient isoform of the tumor suppressor p53. We discovered that Δ40p53, in addition to being highly expressed in embryonic stem cells (ESCs), is the major p53 isoform during early stages of embryogenesis in the mouse. By altering the dose of Δ40p53 in ESCs, we identified a critical role for this isoform in maintaining the ESC state. Haploinsufficiency for Δ40p53 causes a loss of pluripotency in ESCs and acquisition of a somatic cell cycle, while increased dosage of Δ40p53 prolongs pluripotency and inhibits progression to a more differentiated state. Δ40p53 controls the switch from pluripotent ESCs to differentiated somatic cells by controlling the activity of full-length p53 at critical targets such as Nanog and the IGF-1 receptor (IGF-1R). The IGF axis plays a central role in the switch between pluripotency and differentiation in ESCs-and Δ40p53, by controlling the level of the IGF-1R, acts as a master regulator of this switch. We propose that this is the primary function of Δ40p53 in cells of the early embryo and stem cells, which are the only normal cells in which this isoform is expressed.
Figures





References
-
- Aladjem MI, Spike BT, Rodewald LW, Hope TJ, Klemm M, Jaenisch R, Wahl GM 1998. ES cells do not activate p53-dependent stress responses and undergo p53-independent apoptosis in response to DNA damage. Curr Biol 8: 145–155 - PubMed
-
- Bendall SC, Stewart MH, Menendez P, George D, Vijayaragavan K, Werbowetski-Ogilvie T, Ramos-Mejia V, Rouleau A, Yang J, Bosse M, et al. 2007. IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature 448: 1015–1021 - PubMed
-
- Corbet SW, Clarke AR, Gledhill S, Wyllie AH 1999. P53-dependent and -independent links between DNA-damage, apoptosis and mutation frequency in ES cells. Oncogene 18: 1537–1544 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
