The Lats2 tumor suppressor augments p53-mediated apoptosis by promoting the nuclear proapoptotic function of ASPP1
- PMID: 21041410
- PMCID: PMC2964752
- DOI: 10.1101/gad.1954410
The Lats2 tumor suppressor augments p53-mediated apoptosis by promoting the nuclear proapoptotic function of ASPP1
Abstract
Apoptosis is an important mechanism to eliminate potentially tumorigenic cells. The tumor suppressor p53 plays a pivotal role in this process. Many tumors harbor mutant p53, but others evade its tumor-suppressive effects by altering the expression of proteins that regulate the p53 pathway. ASPP1 (apoptosis-stimulating protein of p53-1) is a key mediator of the nuclear p53 apoptotic response. Under basal conditions, ASPP1 is cytoplasmic. We report that, in response to oncogenic stress, the tumor suppressor Lats2 (large tumor suppressor 2) phosphorylates ASPP1 and drives its translocation into the nucleus. Together, Lats2 and ASPP1 shunt p53 to proapoptotic promoters and promote the death of polyploid cells. These effects are overridden by the Yap1 (Yes-associated protein 1) oncoprotein, which disrupts Lats2-ASPP1 binding and antagonizes the tumor-suppressing function of the Lats2/ASPP1/p53 axis.
Figures
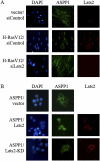
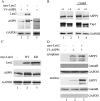
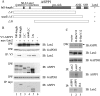

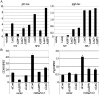


Comment in
-
YAP tips the balance.Nat Rev Cancer. 2010 Dec;10(12):811. doi: 10.1038/nrc2973. Nat Rev Cancer. 2010. PMID: 21155175 No abstract available.
References
-
- Agirre X, Roman-Gomez J, Jimenez-Velasco A, Garate L, Montiel-Duarte C, Navarro G, Vazquez I, Zalacain M, Calasanz MJ, Heiniger A, et al. 2006. ASPP1, a common activator of TP53, is inactivated by aberrant methylation of its promoter in acute lymphoblastic leukemia. Oncogene 25: 1862–1870 - PubMed
-
- Bergamaschi D, Samuels Y, O'Neil NJ, Trigiante G, Crook T, Hsieh JK, O'Connor DJ, Zhong S, Campargue I, Tomlinson ML, et al. 2003. iASPP oncoprotein is a key inhibitor of p53 conserved from worm to human. Nat Genet 33: 162–167 - PubMed
-
- Chen CF, Yeh SH, Chen DS, Chen PJ, Jou YS 2005. Molecular genetic evidence supporting a novel human hepatocellular carcinoma tumor suppressor locus at 13q12.11. Genes Chromosomes Cancer 44: 320–328 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
