A fadD mutant of Vibrio cholerae is impaired in the production of virulence factors and membrane localization of the virulence regulatory protein TcpP
- PMID: 21041490
- PMCID: PMC3019873
- DOI: 10.1128/IAI.00663-10
A fadD mutant of Vibrio cholerae is impaired in the production of virulence factors and membrane localization of the virulence regulatory protein TcpP
Abstract
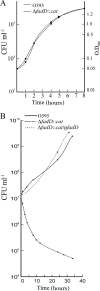
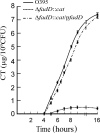
In the enteric pathogen Vibrio cholerae, expression of the major virulence factors is controlled by the hierarchical expression of several regulatory proteins comprising the ToxR regulon. In this study, we demonstrate that disruption of the fadD gene encoding a long-chain fatty acyl coenzyme A ligase has marked effects on expression of the ToxR virulence regulon, motility, and in vivo lethality of V. cholerae. In the V. cholerae fadD mutant, expression of the major virulence genes ctxAB and tcpA, encoding cholera toxin (CT), and the major subunit of the toxin-coregulated pilus (TCP) was drastically repressed and a growth-phase-dependent reduction in the expression of toxT, encoding the transcriptional activator of ctxAB and tcpA, was observed. Expression of toxT from an inducible promoter completely restored CT to wild-type levels in the V. cholerae fadD mutant, suggesting that FadD probably acts upstream of toxT expression. Expression of toxT is activated by the synergistic effect of two transcriptional regulators, TcpP and ToxR. Reverse transcription-PCR and Western blot analysis indicated that although gene expression and production of both TcpP and ToxR are unaffected in the fadD mutant strain, membrane localization of TcpP, but not ToxR, is severely impaired in the fadD mutant strain from the mid-logarithmic phase of growth. Since the decrease in toxT expression occurred concomitantly with the reduction in membrane localization of TcpP, a direct correlation between the defect in membrane localization of TcpP and reduced toxT expression in the fadD mutant strain is suggested.
Figures








Similar articles
-
Reduced virulence of the Vibrio cholerae fadD mutant is due to induction of the extracytoplasmic stress response.Infect Immun. 2013 Oct;81(10):3935-41. doi: 10.1128/IAI.00722-13. Epub 2013 Aug 5. Infect Immun. 2013. PMID: 23918781 Free PMC article.
-
Independent Promoter Recognition by TcpP Precedes Cooperative Promoter Activation by TcpP and ToxR.mBio. 2021 Oct 26;12(5):e0221321. doi: 10.1128/mBio.02213-21. Epub 2021 Sep 7. mBio. 2021. PMID: 34488449 Free PMC article.
-
Stringent response interacts with the ToxR regulon to regulate Vibrio cholerae virulence factor expression.Arch Microbiol. 2020 Aug;202(6):1359-1368. doi: 10.1007/s00203-020-01847-6. Epub 2020 Mar 10. Arch Microbiol. 2020. PMID: 32157346
-
Regulation of virulence in Vibrio cholerae: the ToxR regulon.Future Microbiol. 2007 Jun;2(3):335-44. doi: 10.2217/17460913.2.3.335. Future Microbiol. 2007. PMID: 17661707 Review.
-
Co-ordinate expression of virulence genes by ToxR in Vibrio cholerae.Mol Microbiol. 1992 Feb;6(4):451-8. doi: 10.1111/j.1365-2958.1992.tb01489.x. Mol Microbiol. 1992. PMID: 1560773 Review.
Cited by
-
An essential host dietary fatty acid promotes TcpH inhibition of TcpP proteolysis promoting virulence gene expression in Vibrio cholerae.mBio. 2024 Aug 14;15(8):e0072124. doi: 10.1128/mbio.00721-24. Epub 2024 Jul 3. mBio. 2024. PMID: 38958446 Free PMC article.
-
The Fatty Acid Regulator FadR Influences the Expression of the Virulence Cascade in the El Tor Biotype of Vibrio cholerae by Modulating the Levels of ToxT via Two Different Mechanisms.J Bacteriol. 2017 Mar 14;199(7):e00762-16. doi: 10.1128/JB.00762-16. Print 2017 Apr 1. J Bacteriol. 2017. PMID: 28115548 Free PMC article.
-
The puzzle of two tandem acyl-CoA ligases of Pseudomonas putida F1.Appl Environ Microbiol. 2024 Nov 20;90(11):e0126724. doi: 10.1128/aem.01267-24. Epub 2024 Oct 15. Appl Environ Microbiol. 2024. PMID: 39404437 Free PMC article.
-
Host cell contact induces expression of virulence factors and VieA, a cyclic di-GMP phosphodiesterase, in Vibrio cholerae.J Bacteriol. 2013 May;195(9):2004-10. doi: 10.1128/JB.02127-12. Epub 2013 Feb 22. J Bacteriol. 2013. PMID: 23435982 Free PMC article.
-
In silico analysis of class I adenylate-forming enzymes reveals family and group-specific conservations.PLoS One. 2018 Sep 4;13(9):e0203218. doi: 10.1371/journal.pone.0203218. eCollection 2018. PLoS One. 2018. PMID: 30180199 Free PMC article.
References
-
- Barber, C. E., J. L. Tang, J. X. Feng, M. Q. Pan, T. J. G. Wilson, H. Slater, et al. 1997. A novel regulatory system required for pathogenicity of Xanthomonas campestris is mediated by a small diffusible signal molecule. Mol. Microbiol. 24:555-566. - PubMed
-
- Black, P. N., C. C. DiRusso, A. K. Metzger, and T. L. Heimert. 1992. Cloning, sequencing, and expression of the fadD gene of Escherichia coli encoding acyl coenzyme A synthetase. J. Biol. Chem. 267:25513-25520. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

