Experimental human cytomegalovirus latency in CD14+ monocytes
- PMID: 21041645
- PMCID: PMC2993366
- DOI: 10.1073/pnas.1014509107
Experimental human cytomegalovirus latency in CD14+ monocytes
Abstract
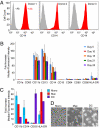
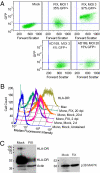
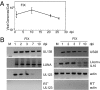
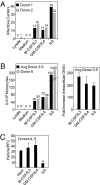
CD14(+) monocytes are a reservoir for latent human cytomegalovirus, and virus replication is reactivated during their differentiation to macrophages or dendritic cells. It has not been clear whether the virus can establish latency upon direct infection of monocytes or whether it must first become quiescent in a progenitor cell that subsequently differentiates to generate a monocyte. We report that infection of primary human monocytes with a clinical strain of human cytomegalovirus exhibits the hallmarks of latency. We established conditions for culturing monocytes that prevent differentiation for at least 25 d, as evidenced by cell surface marker expression. Infection of these monocytes with the FIX clinical strain resulted in transient accumulation of many viral lytic RNAs and sustained expression of four previously described latency-associated transcripts. The amount of viral DNA remained constant after infection, and cell surface and total HLA-DR proteins were substantially reduced on a continuing basis after infection. When treated with cytokine mixtures that stimulate differentiation to a macrophage or dendritic cell phenotype, infected monocytes reactivated virus replication and produced infectious progeny. Treatment of infected monocytes with IL-6 alone also was sufficient for reactivation, and the particles produced after exposure to this cytokine were about fivefold more infectious than virions produced by other treatments. We propose that in vivo microenvironments influence not only the efficiency of reactivation but also the infectivity of the virions produced from latently infected monocytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Mocarski ES, Shenk T, Pass RF. Cytomegaloviruses. In: Knipe DM, Howley PM, editors. Fields Virology. 5th Ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2007. pp. 2701–2772.
-
- Sinclair J. Human cytomegalovirus: Latency and reactivation in the myeloid lineage. J Clin Virol. 2008;41:180–185. - PubMed
-
- Schrier RD, Nelson JA, Oldstone MB. Detection of human cytomegalovirus in peripheral blood lymphocytes in a natural infection. Science. 1985;230:1048–1051. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

