Role for mammalian target of rapamycin complex 1 signaling in neuroadaptations underlying alcohol-related disorders
- PMID: 21041654
- PMCID: PMC2993345
- DOI: 10.1073/pnas.1005554107
Role for mammalian target of rapamycin complex 1 signaling in neuroadaptations underlying alcohol-related disorders
Abstract
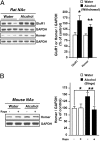
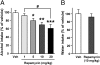
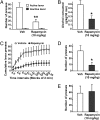
Alcohol addiction is a chronically relapsing disorder that includes certain maladaptive learning and memory. The serine and threonine kinase complex, mammalian target of rapamycin complex 1 (mTORC1), has been implicated in synaptic plasticity, learning, and memory by controlling protein translation. Here we show that administration of alcohol and excessive voluntary consumption of alcohol induce the activation of the mTORC1-mediated signaling pathway in the nucleus accumbens (NAc) of rodents. We further show that the protein expression levels of GluR1 and Homer, two synaptic proteins whose translation has been shown to be modulated by mTORC1, are up-regulated in the NAc of rodents with a history of excessive alcohol consumption. In addition, our results document that the Food and Drug Administration-approved inhibitor of mTORC1, rapamycin, decreases expression of alcohol-induced locomotor sensitization and place preference, as well as excessive alcohol intake and seeking in preclinical rodent models of alcohol abuse. Together, our results suggest that mTORC1 within the NAc is a contributor to molecular mechanisms underlying alcohol-drinking behaviors. Furthermore, despite its massive health and socioeconomic impact worldwide, pharmacotherapies for alcohol abuse and addiction remain limited. Our data therefore put forward the possibility that targeting the mTORC1 signaling cascade is an innovative and valuable strategy for the treatment of alcohol use and abuse disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
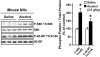



Comment in
-
Addiction: a sobering thought.Nat Rev Neurosci. 2011 Jan;12(1):4. doi: 10.1038/nrn2964. Nat Rev Neurosci. 2011. PMID: 21218569 No abstract available.
References
-
- Spanagel R. Alcoholism: A systems approach from molecular physiology to addictive behavior. Physiol Rev. 2009;89:649–705. - PubMed
-
- Hyman SE. Addiction: A disease of learning and memory. Am J Psychiatry. 2005;162:1414–1422. - PubMed
-
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: The role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. - PubMed
-
- Kelley AE. Memory and addiction: Shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

