Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts
- PMID: 21041659
- PMCID: PMC2993333
- DOI: 10.1073/pnas.1013805107
Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts
Abstract
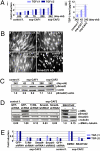
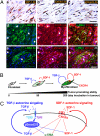
Much interest is currently focused on the emerging role of tumor-stroma interactions essential for supporting tumor progression. Carcinoma-associated fibroblasts (CAFs), frequently present in the stroma of human breast carcinomas, include a large number of myofibroblasts, a hallmark of activated fibroblasts. These fibroblasts have an ability to substantially promote tumorigenesis. However, the precise cellular origins of CAFs and the molecular mechanisms by which these cells evolve into tumor-promoting myofibroblasts remain unclear. Using a coimplantation breast tumor xenograft model, we show that resident human mammary fibroblasts progressively convert into CAF myofibroblasts during the course of tumor progression. These cells increasingly acquire two autocrine signaling loops, mediated by TGF-β and SDF-1 cytokines, which both act in autostimulatory and cross-communicating fashions. These autocrine-signaling loops initiate and maintain the differentiation of fibroblasts into myofibroblasts and the concurrent tumor-promoting phenotype. Collectively, these findings indicate that the establishment of the self-sustaining TGF-β and SDF-1 autocrine signaling gives rise to tumor-promoting CAF myofibroblasts during tumor progression. This autocrine-signaling mechanism may prove to be an attractive therapeutic target to block the evolution of tumor-promoting CAFs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Sappino AP, Skalli O, Jackson B, Schürch W, Gabbiani G. Smooth-muscle differentiation in stromal cells of malignant and non-malignant breast tissues. Int J Cancer. 1988;41:707–712. - PubMed
-
- Kellermann MG, et al. Mutual paracrine effects of oral squamous cell carcinoma cells and normal oral fibroblasts: Induction of fibroblast to myofibroblast transdifferentiation and modulation of tumor cell proliferation. Oral Oncol. 2008;44:509–517. - PubMed
-
- Cardone A, Tolino A, Zarcone R, Borruto Caracciolo G, Tartaglia E. Prognostic value of desmoplastic reaction and lymphocytic infiltration in the management of breast cancer. Panminerva Med. 1997;39:174–177. - PubMed
-
- Maeshima AM, et al. Modified scar grade: A prognostic indicator in small peripheral lung adenocarcinoma. Cancer. 2002;95:2546–2554. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

