Titin is a target of matrix metalloproteinase-2: implications in myocardial ischemia/reperfusion injury
- PMID: 21041693
- PMCID: PMC3057897
- DOI: 10.1161/CIRCULATIONAHA.109.930222
Titin is a target of matrix metalloproteinase-2: implications in myocardial ischemia/reperfusion injury
Abstract
Background: Titin is the largest mammalian (≈3000 to 4000 kDa) and myofilament protein that acts as a molecular spring in the cardiac sarcomere and determines systolic and diastolic function. Loss of titin in ischemic hearts has been reported, but the mechanism of titin degradation is not well understood. Matrix metalloproteinase-2 (MMP-2) is localized to the cardiac sarcomere and, on activation in ischemia/reperfusion injury, proteolyzes specific myofilament proteins. Here we determine whether titin is an intracellular substrate for MMP-2 and if its degradation during ischemia/reperfusion contributes to cardiac contractile dysfunction.
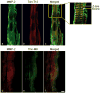
Methods and results: Immunohistochemistry and confocal microscopy in rat and human hearts showed discrete colocalization between MMP-2 and titin in the Z-disk region of titin and that MMP-2 is localized mainly to titin near the Z disk of the cardiac sarcomere. Both purified titin and titin in skinned cardiomyocytes were proteolyzed when incubated with MMP-2 in a concentration-dependent manner, and this was prevented by MMP inhibitors. Isolated rat hearts subjected to ischemia/reperfusion injury showed cleavage of titin in ventricular extracts by gel electrophoresis, which was confirmed by reduced titin immunostaining in tissue sections. Inhibition of MMP activity with ONO-4817 prevented ischemia/reperfusion-induced titin degradation and improved the recovery of myocardial contractile function. Titin degradation was also reduced in hearts from MMP-2 knockout mice subjected to ischemia/reperfusion in vivo compared with wild-type controls.
Conclusion: MMP-2 localizes to titin at the Z-disk region of the cardiac sarcomere and contributes to titin degradation in myocardial ischemia/reperfusion injury.
Figures





Comment in
-
Molecular giant vulnerable to oxidative damage: titin joins the club of proteins degraded by matrix metalloproteinase-2.Circulation. 2010 Nov 16;122(20):2002-4. doi: 10.1161/CIRCULATIONAHA.110.985317. Epub 2010 Nov 1. Circulation. 2010. PMID: 21041688 No abstract available.
References
-
- Birkedal-Hansen H. Proteolytic remodeling of extracellular matrix. Curr Opin Cell Biol. 1995;7:728–735. - PubMed
-
- Yasmin W, Strynadka KD, Schulz R. Generation of peroxynitrite contributes to ischemia-reperfusion injury in isolated rat hearts. Cardiovasc Res. 1997;33:422–432. - PubMed
-
- Okamoto T, Akaike T, Sawa T, Miyamoto Y, van der Vliet A, Maeda H. Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. J Biol Chem. 2001;276:29596–29602. - PubMed
-
- Viappiani S, Nicolescu AC, Holt A, Sawicki G, Crawford BD, Leon H, van Mulligen T, Schulz R. Activation and modulation of 72kDa matrix metalloproteinase-2 by peroxynitrite and glutathione. Biochem Pharmacol. 2009;77:826–834. - PubMed
-
- Woessner JF., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145–2154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

