The FLARE intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in perforator flap breast reconstruction
- PMID: 21042103
- PMCID: PMC2974179
- DOI: 10.1097/PRS.0b013e3181f059c7
The FLARE intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in perforator flap breast reconstruction
Abstract
Background: The ability to determine flap perfusion in reconstructive surgery is still primarily based on clinical examination. In this study, the authors demonstrate the use of an intraoperative, near-infrared fluorescence imaging system for evaluation of perforator location and flap perfusion.
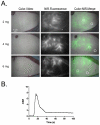
Methods: Indocyanine green was injected intravenously in six breast cancer patients undergoing a deep inferior epigastric perforator flap breast reconstruction after mastectomy. Three dose levels of indocyanine green were assessed using the fluorescence-assisted resection and exploration (FLARE) imaging system. This system uses light-emitting diodes for fluorescence excitation, which is different from current commercially available systems. In this pilot study, the operating surgeons were blinded to the imaging results.
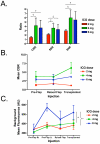
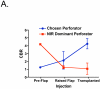
Results: Use of the FLARE system was successful in all six study subjects, with no complications or sequelae. Among the three dose levels, 4 mg per injection resulted in the highest observed contrast-to-background ratio, signal-to-background ratio, and signal-to-noise ratio. However, because of small sample size, the authors did not have sufficient power to detect statistical significance for these pairwise comparisons at the multiple-comparison adjusted type I error of 0.017. Six milligrams per injection provided a similar contrast-to-background ratio but also a higher residual background signal.
Conclusion: Based on this pilot study, the authors conclude that near-infrared assessment of perforator flap breast reconstruction is feasible with a light-emitting diode-based system, and that a dose of 4 mg of indocyanine green per injection yields the best observed contrast-to-background ratio compared with a dose of 2 or 6 mg for assessment of flap perfusion.
Trial registration: ClinicalTrials.gov NCT00952107.
Figures


References
-
- Giunta RE, Geisweid A, Feller AM. The value of preoperative Doppler sonography for planning free perforator flaps. Plast Reconstr Surg. 2000;105:2381–2386. - PubMed
-
- Masia J, Clavero JA, Larranaga JR, et al. Multidetector-row computed tomography in the planning of abdominal perforator flaps. J Plast Reconstr Aesthet Surg. 2006;59:594–599. - PubMed
-
- Rozen WM, Stella DL, Phillips TJ, et al. Magnetic resonance angiography in the preoperative planning of DIEA perforator flaps. Plast Reconstr Surg. 2008;122:222e–223e. - PubMed
-
- Saint-Cyr M, Schaverien M, Arbique G, et al. Three- and four-dimensional computed tomographic angiography and venography for the investigation of the vascular anatomy and perfusion of perforator flaps. Plast Reconstr Surg. 2008;121:772–780. - PubMed
-
- Mathes DW, Neligan PC. Current techniques in preoperative imaging for abdomen-based perforator flap microsurgical breast reconstruction. J Reconstr Microsurg. 2010;26:3–10. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

