Familial glucocorticoid receptor haploinsufficiency by non-sense mediated mRNA decay, adrenal hyperplasia and apparent mineralocorticoid excess
- PMID: 21042587
- PMCID: PMC2962642
- DOI: 10.1371/journal.pone.0013563
Familial glucocorticoid receptor haploinsufficiency by non-sense mediated mRNA decay, adrenal hyperplasia and apparent mineralocorticoid excess
Abstract
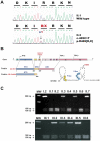
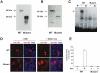
Primary glucocorticoid resistance (OMIM 138040) is a rare hereditary disease that causes a generalized partial insensitivity to glucocorticoid action, due to genetic alterations of the glucocorticoid receptor (GR). Investigation of adrenal incidentalomas led to the discovery of a family (eight affected individuals spanning three generations), prone to cortisol resistance, bilateral adrenal hyperplasia, arterial hypertension and hypokalemia. This phenotype exacerbated over time, cosegregates with the first heterozygous nonsense mutation p.R469[R,X] reported to date for the GR, replacing an arginine (CGA) by a stop (TGA) at amino-acid 469 in the second zinc finger of the DNA-binding domain of the receptor. In vitro, this mutation leads to a truncated 50-kDa GR lacking hormone and DNA binding capacity, devoid of hormone-dependent nuclear translocation and transactivation properties. In the proband's fibroblasts, we provided evidence for the lack of expression of the defective allele in vivo. The absence of detectable mutated GR mRNA was accompanied by a 50% reduction in wild type GR transcript and protein. This reduced GR expression leads to a significantly below-normal induction of glucocorticoid-induced target genes, FKBP5 in fibroblasts. We demonstrated that the molecular mechanisms of glucocorticoid signaling dysfunction involved GR haploinsufficiency due to the selective degradation of the mutated GR transcript through a nonsense-mediated mRNA Decay that was experimentally validated on emetine-treated propositus' fibroblasts. GR haploinsufficiency leads to hypertension due to illicit occupation of renal mineralocorticoid receptor by elevated cortisol rather than to increased mineralocorticoid production reported in primary glucocorticoid resistance. Indeed, apparent mineralocorticoid excess was demonstrated by a decrease in urinary tetrahydrocortisone-tetrahydrocortisol ratio in affected patients, revealing reduced glucocorticoid degradation by renal activity of the 11β-hydroxysteroid dehydrogenase type 2, a GR regulated gene. We propose thus that GR haploinsufficiency compromises glucocorticoid sensitivity and may represent a novel genetic cause of subclinical hypercortisolism, incidentally revealed bilateral adrenal hyperplasia and mineralocorticoid-independent hypertension.
Conflict of interest statement
Figures


References
-
- Lu NZ, Wardell SE, Burnstein KL, Defranco D, Fuller PJ, et al. International Union of Pharmacology. LXV. The pharmacology and classification of the nuclear receptor superfamily: glucocorticoid, mineralocorticoid, progesterone, and androgen receptors. Pharmacol Rev. 2006;58:782–797. - PubMed
-
- Lu NZ, Cidlowski JA. The origin and functions of multiple human glucocorticoid receptor isoforms. Ann N Y Acad Sci. 2004;1024:102–123. - PubMed
-
- Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids–new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

