Functional invariant NKT cells in pig lungs regulate the airway hyperreactivity: a potential animal model
- PMID: 21042929
- PMCID: PMC4450678
- DOI: 10.1007/s10875-010-9476-4
Functional invariant NKT cells in pig lungs regulate the airway hyperreactivity: a potential animal model
Abstract
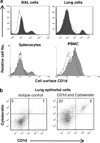
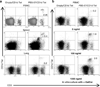
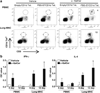
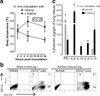
Important roles played by invariant natural killer T (iNKT) cells in asthma pathogenesis have been demonstrated. We identified functional iNKT cells and CD1d molecules in pig lungs. Pig iNKT cells cultured in the presence of α-GalCer proliferated and secreted Th1 and Th2 cytokines. Like in other animal models, direct activation of pig lung iNKT cells using α-GalCer resulted in acute airway hyperreactivity (AHR). Clinically, acute AHR-induced pigs had increased respiratory rate, enhanced mucus secretion in the airways, fever, etc. In addition, we observed petechial hemorrhages, infiltration of CD4(+) cells, and increased Th2 cytokines in AHR-induced pig lungs. Ex vivo proliferated iNKT cells of asthma induced pigs in the presence of C-glycoside analogs of α-GalCer had predominant Th2 phenotype and secreted more of Th2 cytokine, IL-4. Thus, baby pigs may serve as a useful animal model to study iNKT cell-mediated AHR caused by various environmental and microbial CD1d-specific glycolipid antigens.
Figures

Similar articles
-
A CD1d-dependent antagonist inhibits the activation of invariant NKT cells and prevents development of allergen-induced airway hyperreactivity.J Immunol. 2010 Feb 15;184(4):2107-15. doi: 10.4049/jimmunol.0901208. Epub 2010 Jan 18. J Immunol. 2010. PMID: 20083656 Free PMC article.
-
The development of airway hyperreactivity in T-bet-deficient mice requires CD1d-restricted NKT cells.J Immunol. 2009 Mar 1;182(5):3252-61. doi: 10.4049/jimmunol.0803339. J Immunol. 2009. PMID: 19234223
-
Activation of nonclassical CD1d-restricted NK T cells induces airway hyperreactivity in beta 2-microglobulin-deficient mice.J Immunol. 2008 Oct 1;181(7):4560-4569. doi: 10.4049/jimmunol.181.7.4560. J Immunol. 2008. PMID: 18802058 Free PMC article.
-
Tailored design of NKT-stimulatory glycolipids for polarization of immune responses.J Biomed Sci. 2017 Mar 23;24(1):22. doi: 10.1186/s12929-017-0325-0. J Biomed Sci. 2017. PMID: 28335781 Free PMC article. Review.
-
Invariant natural killer T (iNKT) cells in asthma: a novel insight into the pathogenesis of asthma and the therapeutic implication of glycolipid ligands for allergic diseases.Allergol Int. 2007 Mar;56(1):7-14. doi: 10.2332/allergolint.R-06-137. Epub 2007 Jan 29. Allergol Int. 2007. PMID: 17259804 Review.
Cited by
-
Preclinical toxicological assessment of an α-galactosylceramide-adjuvanted mRNA cancer vaccine in Wistar Han rats and domestic pigs.Mol Ther Methods Clin Dev. 2025 May 19;33(2):101493. doi: 10.1016/j.omtm.2025.101493. eCollection 2025 Jun 12. Mol Ther Methods Clin Dev. 2025. PMID: 40519325 Free PMC article.
-
Intranasal delivery of whole cell lysate of Mycobacterium tuberculosis induces protective immune responses to a modified live porcine reproductive and respiratory syndrome virus vaccine in pigs.Vaccine. 2011 May 23;29(23):4067-76. doi: 10.1016/j.vaccine.2011.03.005. Epub 2011 Apr 9. Vaccine. 2011. PMID: 21419164 Free PMC article.
-
Crosstalk between trace elements and T-cell immunity during early-life health in pigs.Sci China Life Sci. 2023 Sep;66(9):1994-2005. doi: 10.1007/s11427-022-2339-0. Epub 2023 Jun 6. Sci China Life Sci. 2023. PMID: 37300752 Review.
-
Modulation of Immune Responses to Influenza A Virus Vaccines by Natural Killer T Cells.Front Immunol. 2020 Oct 20;11:2172. doi: 10.3389/fimmu.2020.02172. eCollection 2020. Front Immunol. 2020. PMID: 33193296 Free PMC article. Review.
-
The bovine CD1D gene has an unusual gene structure and is expressed but cannot present α-galactosylceramide with a C26 fatty acid.Int Immunol. 2013 Feb;25(2):91-8. doi: 10.1093/intimm/dxs092. Epub 2012 Sep 11. Int Immunol. 2013. PMID: 22968995 Free PMC article.
References
-
- Ibrahim Z, Busch J, Awwad M, Wagner R, Wells K, Cooper DK. Selected physiologic compatibilities and incompatibilities between human and porcine organ systems. Xenotransplantation. 2006;13(6):488–499. - PubMed
-
- Baskerville A. Development of the early lesions in experimental enzootic pneumonia of pigs: an ultrastructural and histological study. Res Vet Sci. 1972;13(6):570–578. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

