A non-human primate model for analysis of safety, persistence, and function of adoptively transferred T cells
- PMID: 21044089
- PMCID: PMC3048898
- DOI: 10.1111/j.1600-0684.2010.00451.x
A non-human primate model for analysis of safety, persistence, and function of adoptively transferred T cells
Abstract
Background: Adoptive immunotherapy with antigen-specific effector T-cell (T(E) ) clones is often limited by poor survival of the transferred cells. We describe here a Macaca nemestrina model for studying transfer of T-cell immunity.
Methods: We derived, expanded, and genetically marked CMV-specific CD8(+) T(E) clones with surface markers expressed on B cells. T(E) cells were adoptively transferred, and toxicity, persistence, retention of introduced cell-surface markers, and phenotype of the persisting T cells were evaluated.
Results: CD8(+) T(E) clones were efficiently isolated from distinct memory precursors and gene-marking with CD19 or CD20 permitted in vivo tracking by quantitative PCR. CD19 was a more stable surface marker for tracking cells in vivo and was used to re-isolate cells for functional analysis. Clonally derived CD8(+) T(E) cells differentiated in vivo to phenotypically and functionally heterogeneous memory T-cell subsets.
Conclusions: These studies demonstrate the utility of Macaca nemestrina for establishing principles for T-cell therapeutics applicable to humans.
© 2010 John Wiley & Sons A/S.
Figures

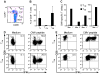

 ) T cells were examined by flow cytometry after staining with mAbs against CD3, CD8, and CD19 or CD20 mAbs. Shown are mean and range of the results with the ΔCD19 (n=7) or CD20 (n=3) marker. (B) In vitro growth of gene-modified TE clones. Left panel: Representative TE clone either unmodified (◇) or ΔCD19+ (◆). Right panel: Representative TE clone either unmodified (◇) or CD20+ (●). Aliquots of T cells unmodified or transduced with ΔCD19+ (left panel) or CD20+ (right panel) were restimulated with anti-CD3/CD28 mAbs, irradiated feeder cells and IL-2, and numeric expansion was measured by counting viable cells on the indicated days. Data are representative of results with ΔCD19+ or CD20+ T cells obtained from each three macaques. (C) Gene-marked CMV-specific CD8+ TE clones retain CMV-specific reactivity. Aliquots of CMV-specific CD8+ TE either unmodified or either ΔCD19+ (left panel) and CD20+ (right panel) were restimulated in vitro and examined in a chromium release assay for recognition of autologous target cells, either unpulsed (□) or pulsed with the CMV cognate peptide at an effector-to-target (E/T) ratio of 10:1 (■), 5:1(
) T cells were examined by flow cytometry after staining with mAbs against CD3, CD8, and CD19 or CD20 mAbs. Shown are mean and range of the results with the ΔCD19 (n=7) or CD20 (n=3) marker. (B) In vitro growth of gene-modified TE clones. Left panel: Representative TE clone either unmodified (◇) or ΔCD19+ (◆). Right panel: Representative TE clone either unmodified (◇) or CD20+ (●). Aliquots of T cells unmodified or transduced with ΔCD19+ (left panel) or CD20+ (right panel) were restimulated with anti-CD3/CD28 mAbs, irradiated feeder cells and IL-2, and numeric expansion was measured by counting viable cells on the indicated days. Data are representative of results with ΔCD19+ or CD20+ T cells obtained from each three macaques. (C) Gene-marked CMV-specific CD8+ TE clones retain CMV-specific reactivity. Aliquots of CMV-specific CD8+ TE either unmodified or either ΔCD19+ (left panel) and CD20+ (right panel) were restimulated in vitro and examined in a chromium release assay for recognition of autologous target cells, either unpulsed (□) or pulsed with the CMV cognate peptide at an effector-to-target (E/T) ratio of 10:1 (■), 5:1( ), 2.5:1 (
), 2.5:1 ( ), or 1.25:1 (
), or 1.25:1 ( ). Data are representative of results with ΔCD19+ or CD20+ T cells from each three macaques. (D, E) Stability of the marker-gene expression in macaque CD8+ T cells. The ΔCD19+ (D) or CD20+ (E) T cells were stimulated with anti-CD3/CD28 mAbs and examined by flow cytometry on day 6 (ii) and 14 (iii) of the stimulation cycle for ΔCD19 or CD20 expression. Unmodified T cells served as negative control (i). Aliquots of T cells were also rested and the ΔCD19 or CD20 expression was assessed by flow cytometry after 4 weeks of rest (iv) or 6 days after restimulation with anti-CD3/CD28 mAbs (v). Inset values show the % of CD3+CD8+ T cells positive for ΔCD19 or CD20 and the MFI is indicated for each time point. Data showing the difference in the stability of expression of ΔCD19 and CD20 at the cell surface is representative for experiments with ΔCD19 and CD20-modified T cells from 3 animals, and was observed in eight ΔCD19+ and CD20+ TE clones.
). Data are representative of results with ΔCD19+ or CD20+ T cells from each three macaques. (D, E) Stability of the marker-gene expression in macaque CD8+ T cells. The ΔCD19+ (D) or CD20+ (E) T cells were stimulated with anti-CD3/CD28 mAbs and examined by flow cytometry on day 6 (ii) and 14 (iii) of the stimulation cycle for ΔCD19 or CD20 expression. Unmodified T cells served as negative control (i). Aliquots of T cells were also rested and the ΔCD19 or CD20 expression was assessed by flow cytometry after 4 weeks of rest (iv) or 6 days after restimulation with anti-CD3/CD28 mAbs (v). Inset values show the % of CD3+CD8+ T cells positive for ΔCD19 or CD20 and the MFI is indicated for each time point. Data showing the difference in the stability of expression of ΔCD19 and CD20 at the cell surface is representative for experiments with ΔCD19 and CD20-modified T cells from 3 animals, and was observed in eight ΔCD19+ and CD20+ TE clones.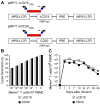
 ) or CD20+ T cells (■) were spiked into aliquots of pre-infusion PBMC (each 105 PBMC/reaction) and examined by real-time qPCR for detection of marker-positive T cells. Data are representative of each 3 assays with ΔCD19+ or CD20+ T cells. (C) Enumeration of transferred ΔCD19+ or CD20+ T cells determined by real-time qPCR for vector sequences. Autologous ΔCD19+ (
) or CD20+ T cells (■) were spiked into aliquots of pre-infusion PBMC (each 105 PBMC/reaction) and examined by real-time qPCR for detection of marker-positive T cells. Data are representative of each 3 assays with ΔCD19+ or CD20+ T cells. (C) Enumeration of transferred ΔCD19+ or CD20+ T cells determined by real-time qPCR for vector sequences. Autologous ΔCD19+ ( ) or CD20+ (■) TCM-derived TE clones were expanded in vitro and transferred to each one of the macaques at a dose of 5×108/kg. DNA was isolated from samples of PBMC obtained before and at indicated time-points after the T-cell infusion and examined by real-time qPCR for detection of marker-positive T cells.
) or CD20+ (■) TCM-derived TE clones were expanded in vitro and transferred to each one of the macaques at a dose of 5×108/kg. DNA was isolated from samples of PBMC obtained before and at indicated time-points after the T-cell infusion and examined by real-time qPCR for detection of marker-positive T cells.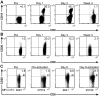

 ) 1.25:1. The transferred ΔCD19+ TE clone served as control. (F) Aliquots of the infused ΔCD19+ TE clone (■) and re-isolated ΔCD19+ TE either obtained from TCM (
) 1.25:1. The transferred ΔCD19+ TE clone served as control. (F) Aliquots of the infused ΔCD19+ TE clone (■) and re-isolated ΔCD19+ TE either obtained from TCM ( ) or TEM (□) were stimulated with medium or CMV peptide-pulsed antigen-presenting cells, and examined by CFC for production of IFN-γ, TNF-α, and IL-2 after gating on CD3+CD8+ T cells.
) or TEM (□) were stimulated with medium or CMV peptide-pulsed antigen-presenting cells, and examined by CFC for production of IFN-γ, TNF-α, and IL-2 after gating on CD3+CD8+ T cells.References
-
- Berger C, Blau CA, Clackson T, Riddell SR, Heimfeld S. CD28 costimulation and immunoaffinity-based selection efficiently generate primary gene-modified T cells for adoptive immunotherapy. Blood. 2003;101:476–484. - PubMed
-
- Berger C, Blau CA, Huang ML, Iuliucci JD, Dalgarno DC, Gaschet J, Heimfeld S, Clackson T, Riddell SR. Pharmacologically regulated Fas-mediated death of adoptively transferred T cells in a nonhuman primate model. Blood. 2004;103:1261–1269. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

